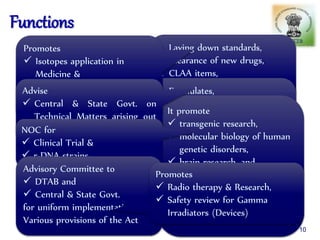
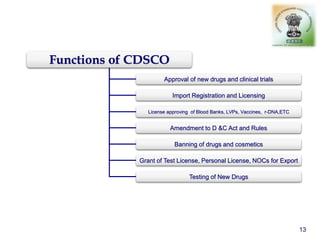
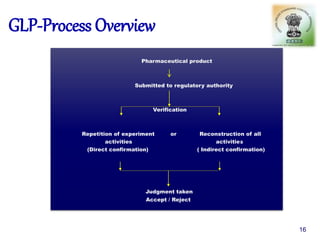
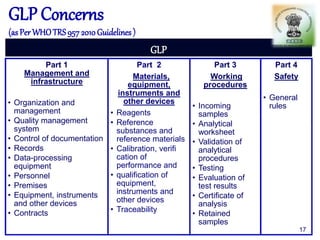
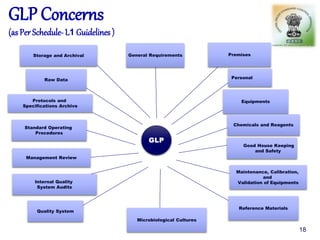
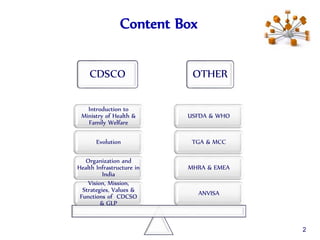
The document outlines the structure and functions of various regulatory agencies involved in drug safety and efficacy, including the CDSCO in India, FDA in the US, and EMEA in Europe. It details the evolution of Indian drug legislation, emphasizing the roles of the Ministry of Health and Family Welfare, as well as the principles of Good Laboratory Practice (GLP) aimed at ensuring the quality of laboratory studies. Additionally, it touches on the significance of Contract Research Organizations (CROs) in the drug development process.







![Indian Drug Regulatory System:
Government of India
Ministry of Health
& Family Welfare
DGHS
Central Drugs
Standard
Control
Organization
(CDSCO)
Ministry of Science
& Technology
Indian
Council of
Medical
Research
(ICMR)
Council of
Scientific &
Industrial
Research
(CSIR)
BARC
(Radioactive)
Ministry of
Chemicals &
Petrochemicals
National
Pharmaceutical
Pricing Authority
(NPPA)
Department of
Chemical &
Petrochemicals
(DCP)
Department of
Pharmaceuticals
Ministry of
Commerce &
Industry
Patent
Office
Dept. of
Commerce &
Pharmexil
Controller
General of
Patent
DGFT
Ministry of
Environment &
Forest
GEAC-
[Genetic
Engineering
Approval
Committee]
Department of
Biotechnology
r-DNA
Advisory
Committee
Review
Committee
Genetic
Manipulation
9](https://image.slidesharecdn.com/regulatorybodiescheck-140701041643-phpapp01/85/Regulatory-bodies-CRO-9-320.jpg)