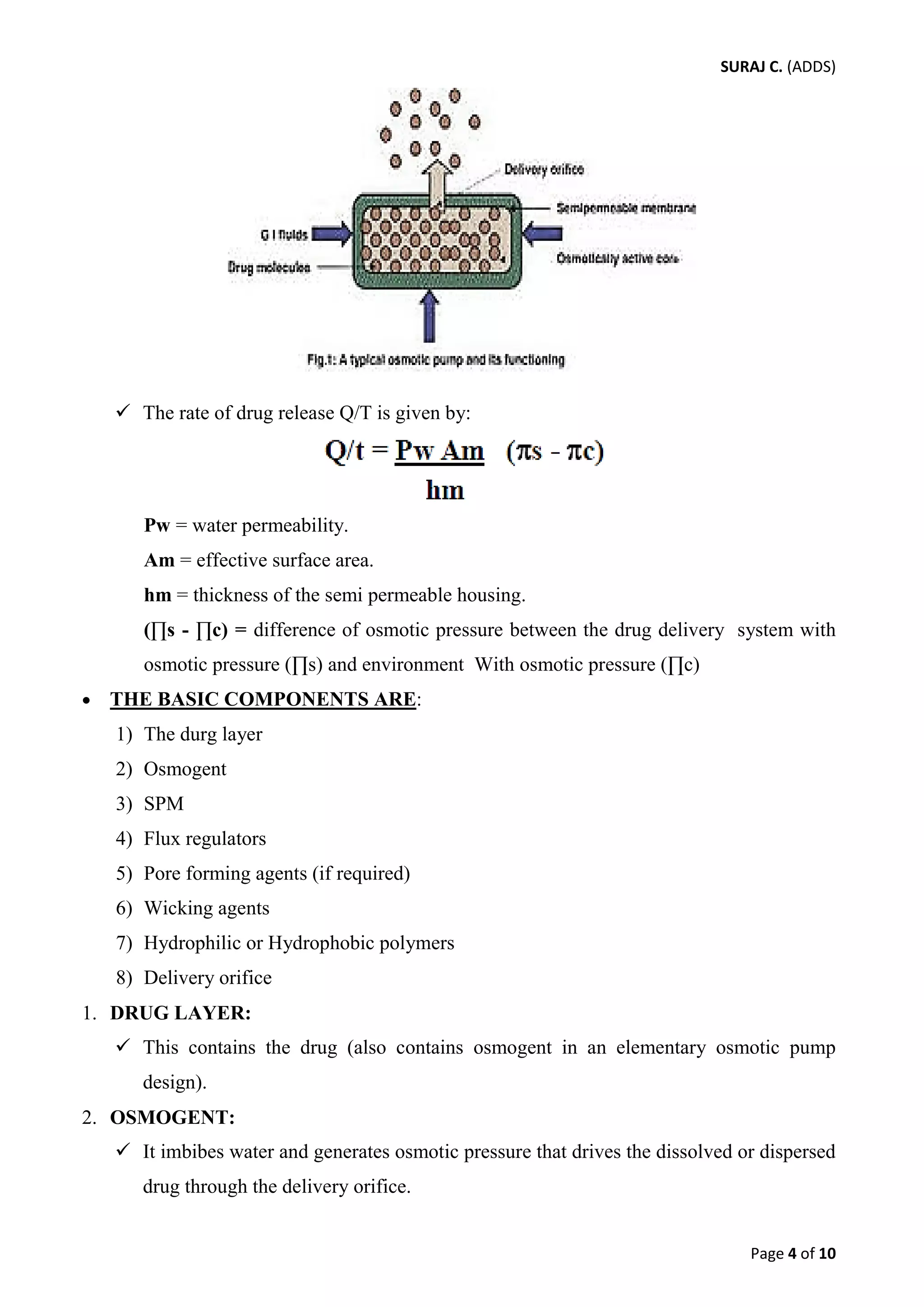
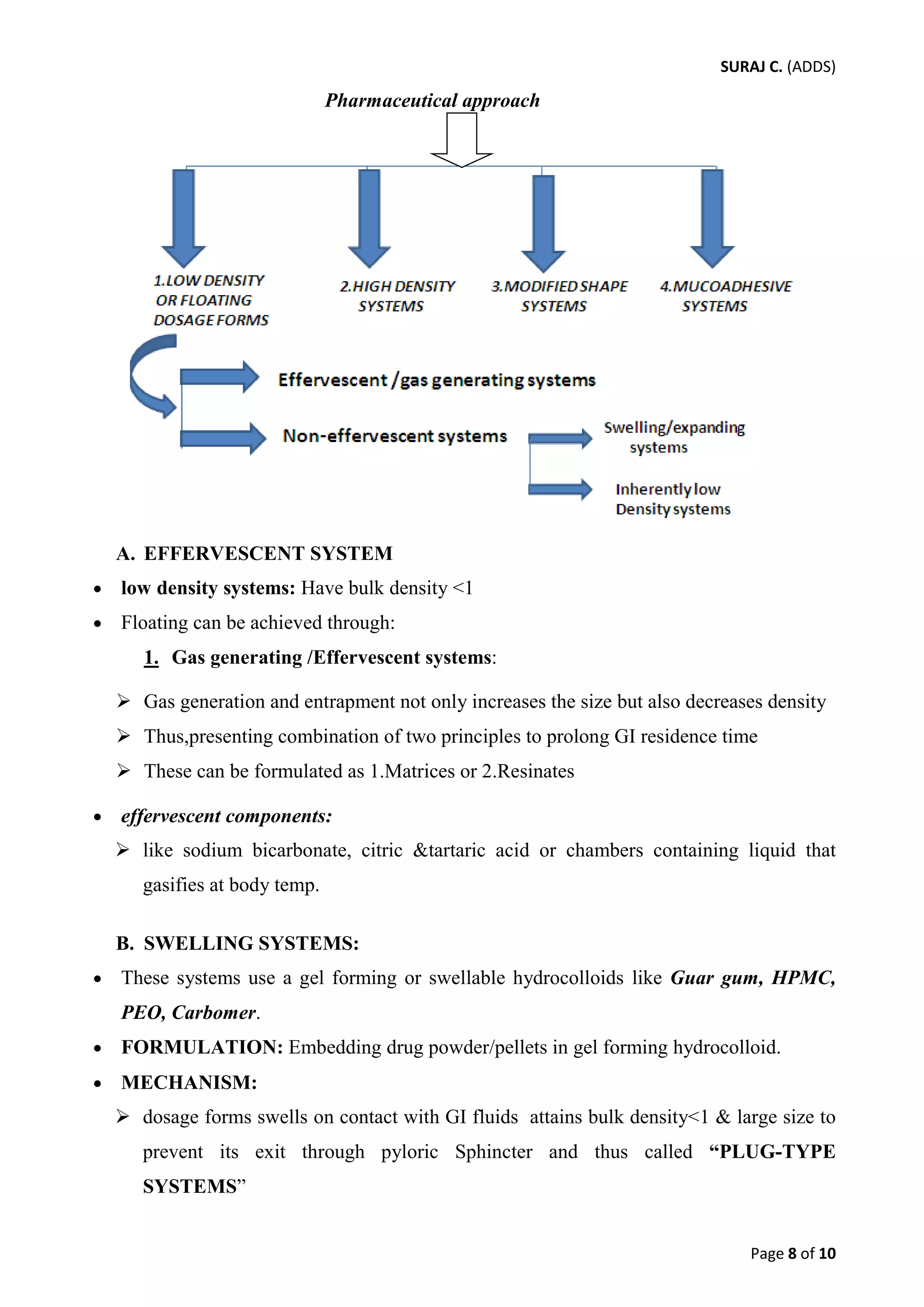
This document provides an overview of three types of rate controlled drug delivery systems: hydrodynamically balanced systems, osmotic pressure controlled systems, and pH dependent/independent systems.
Hydrodynamically balanced systems, also known as floating drug delivery systems, remain buoyant in the stomach for prolonged periods of time to increase bioavailability. Osmotic pressure controlled systems use osmotic pressure to provide zero-order drug release kinetics over extended times. pH dependent systems target drug delivery to specific regions of the GI tract based on pH, while pH independent systems aim to release drugs at a constant rate regardless of varying GI pH.