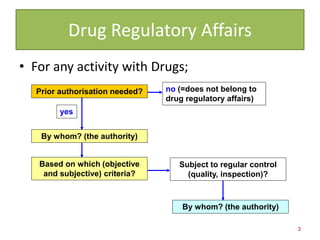
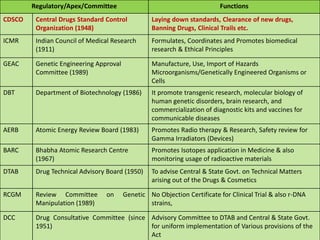
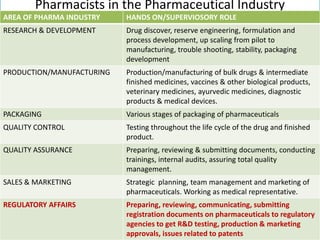
This document discusses the role of pharmacists in drug regulatory affairs in India. It provides an overview of the various drug regulatory authorities and committees in India, including the Central Drugs Standard Control Organization (CDSCO), Drug Technical Advisory Board (DTAB), and Drugs Consultative Committee (DCC). It also outlines some of the key regulations under the Drugs and Cosmetics Act, including licensing requirements for manufacturing, importing, and distributing drugs. Pharmacists can work in areas like quality control, quality assurance, regulatory affairs, and other roles for the pharmaceutical industry and drug regulatory agencies.






![Indian Drug Regulatory System:
Government of India
Ministry of Health &
Family Welfare
DGHS
Central Drugs
Standard Control
Organization
(CDSCO)
Ministry of Science
& Technology
Indian Council
of Medical
Research
(ICMR)
Council of
Scientific &
Industrial
Research
(CSIR)
BARC
(Radioactive)
Ministry of Chemicals
& Petrochemicals
National
Pharmaceutical
Pricing Authority
(NPPA)
Department of
Chemical &
Petrochemicals
(DCP)
Department of
Pharmaceuticals
Ministry of
Commerce &
Industry
Patent
Office
Dept. of
Commerce &
Pharmexil
Controller
General of
Patent
DGFT
Ministry of
Environment &
Forest
GEAC-[Genetic
Engineering
Approval
Committee]
Department of
Biotechnology
r-DNA Advisory
Committee
Review
Committee
Genetic
Manipulation](https://image.slidesharecdn.com/bp603tp4-210326092320/85/Regulatory-Issues-8-320.jpg)









![• Requirements and Guidelines for permission to
manufacture of ASU Drugs for Sale or to
undertake clinical trials [(Proposed) Schedule Z]:
Dept. of AYUSH, Ministry of Health & Family
Welfare, Govt. of India has drafted guidelines on
Good Clinical Practices (GCPs) for Clinical trials on
Ayurveda, Siddha, Unani (ASU) Medicines, which
have been circulated very recently in November
2011](https://image.slidesharecdn.com/bp603tp4-210326092320/85/Regulatory-Issues-21-320.jpg)




