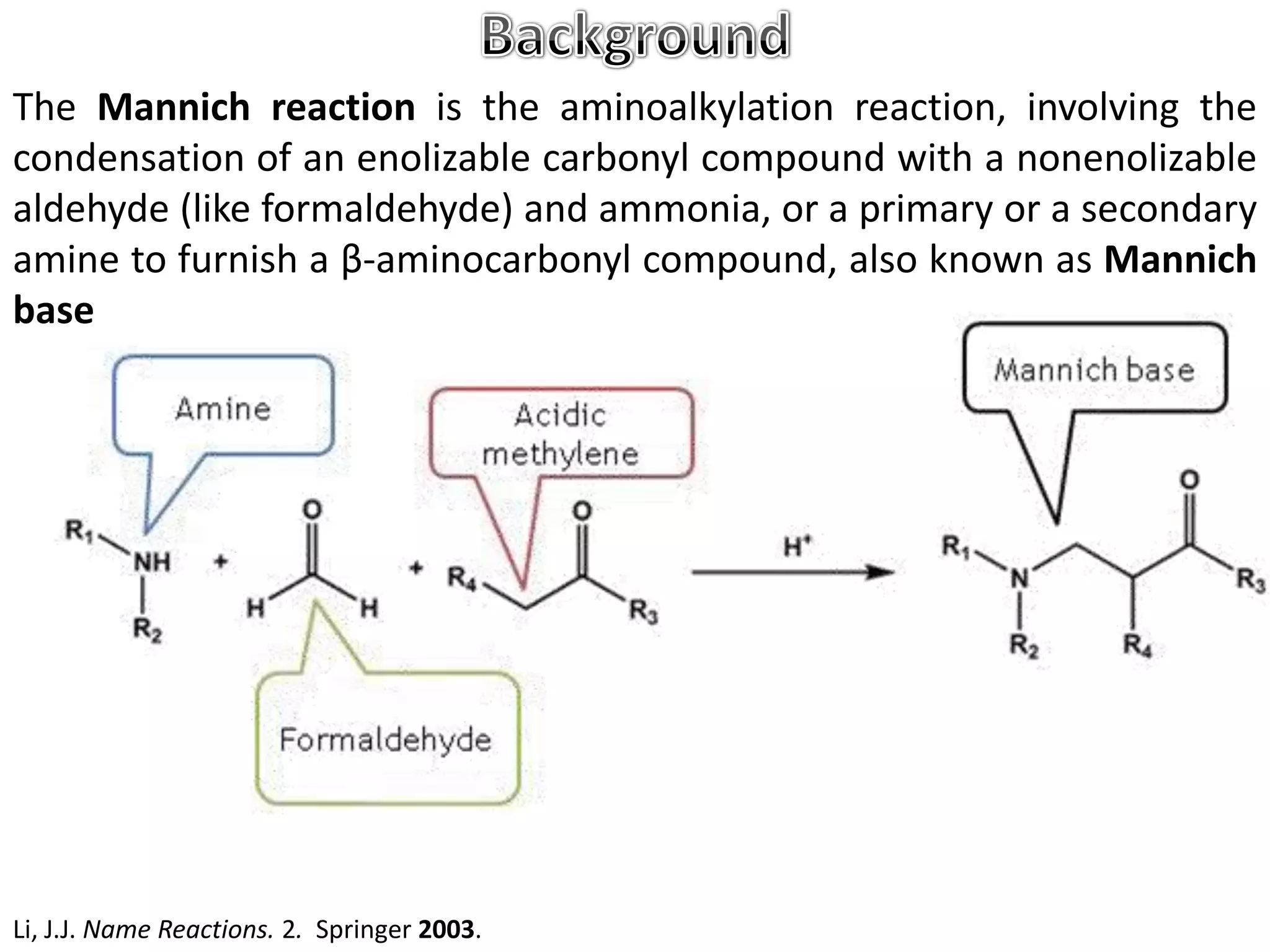
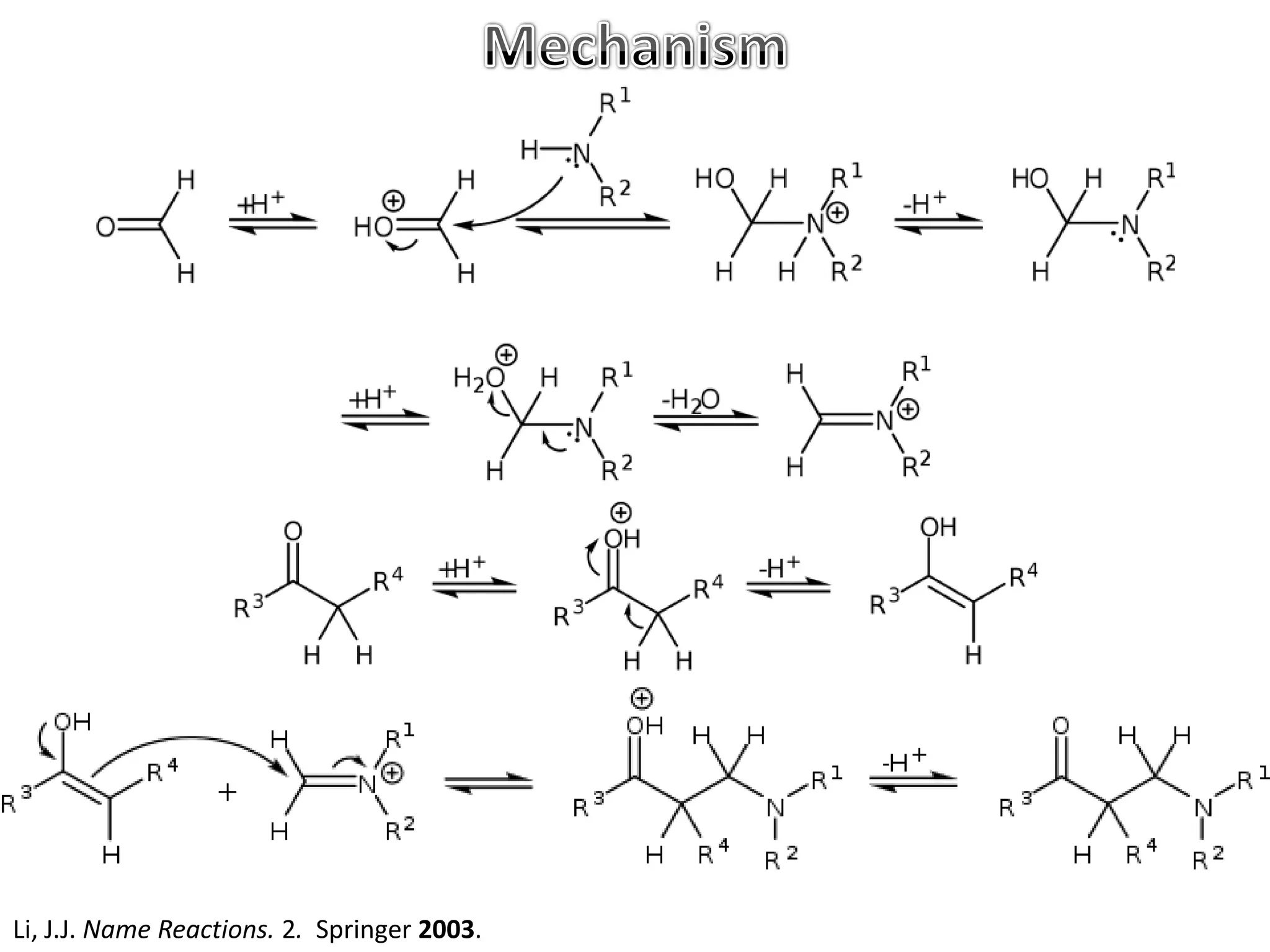
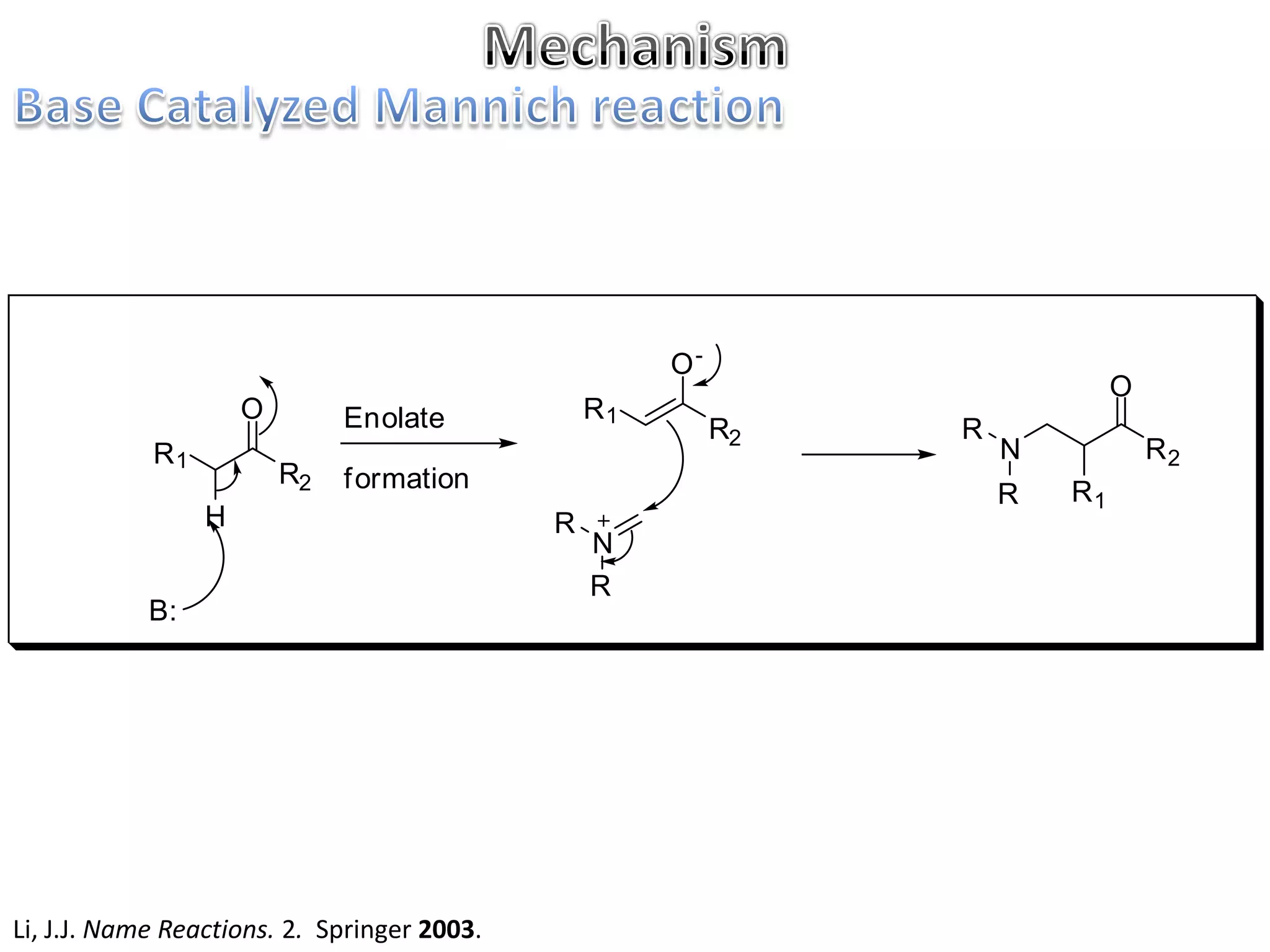
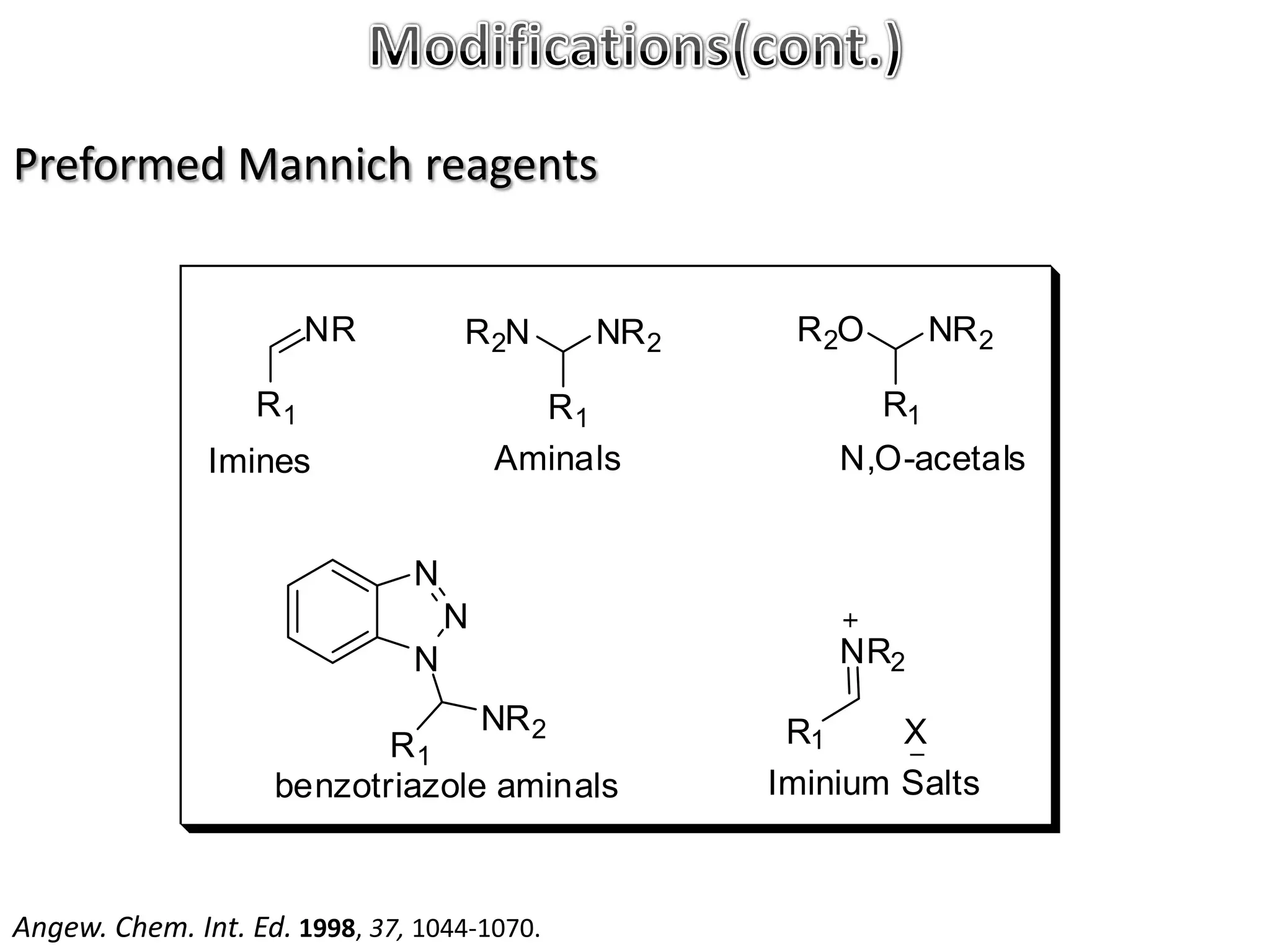
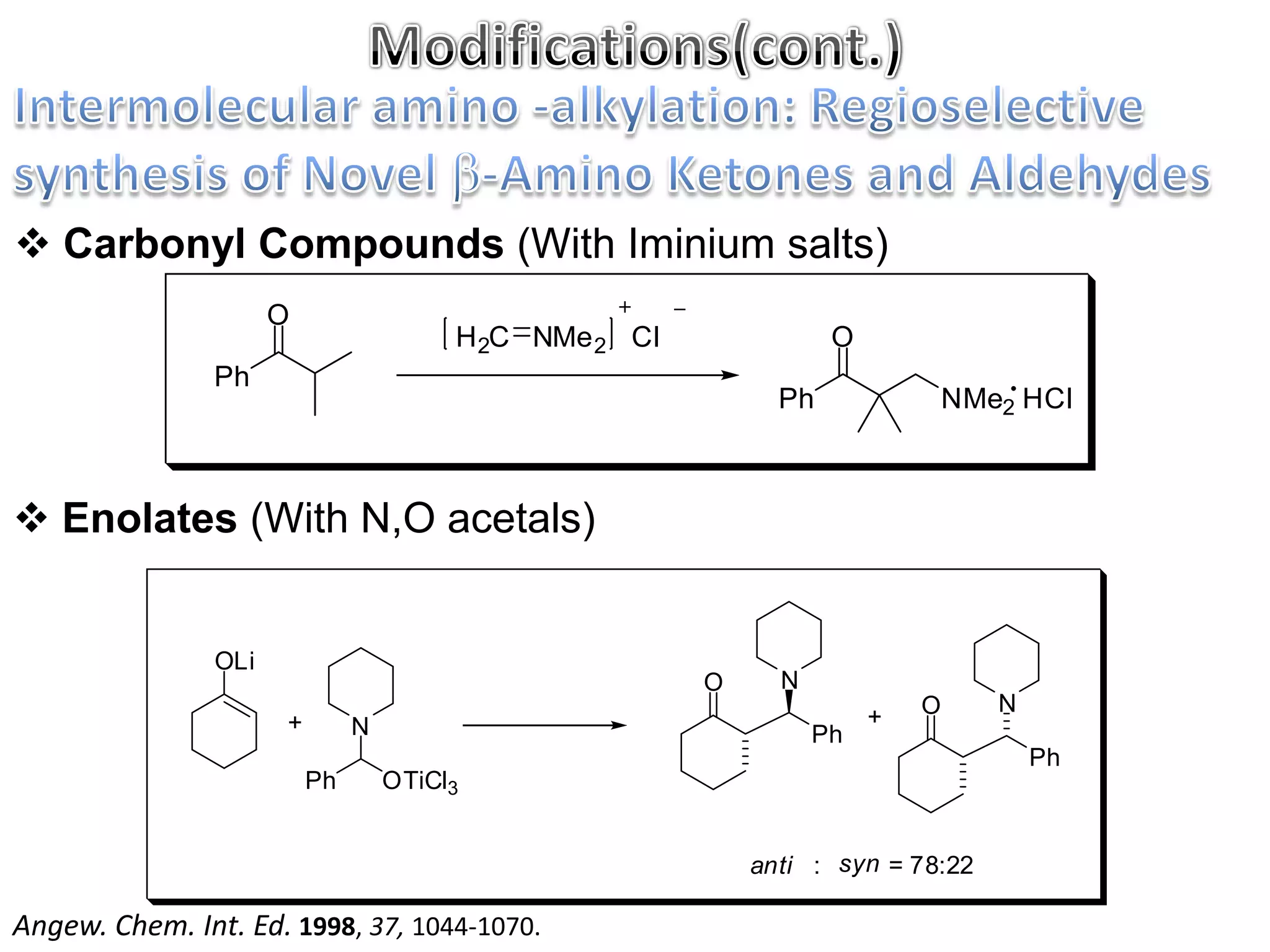
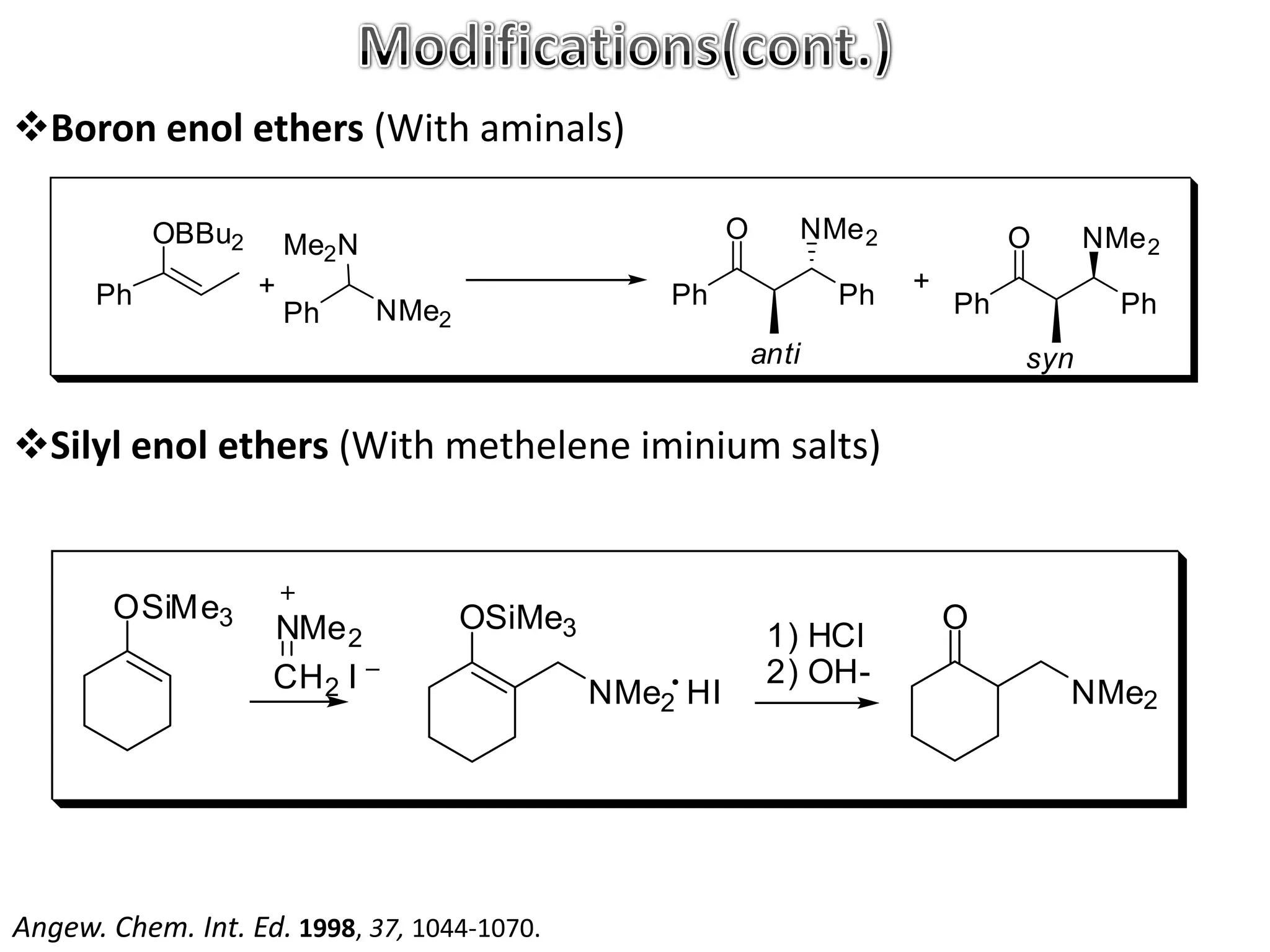
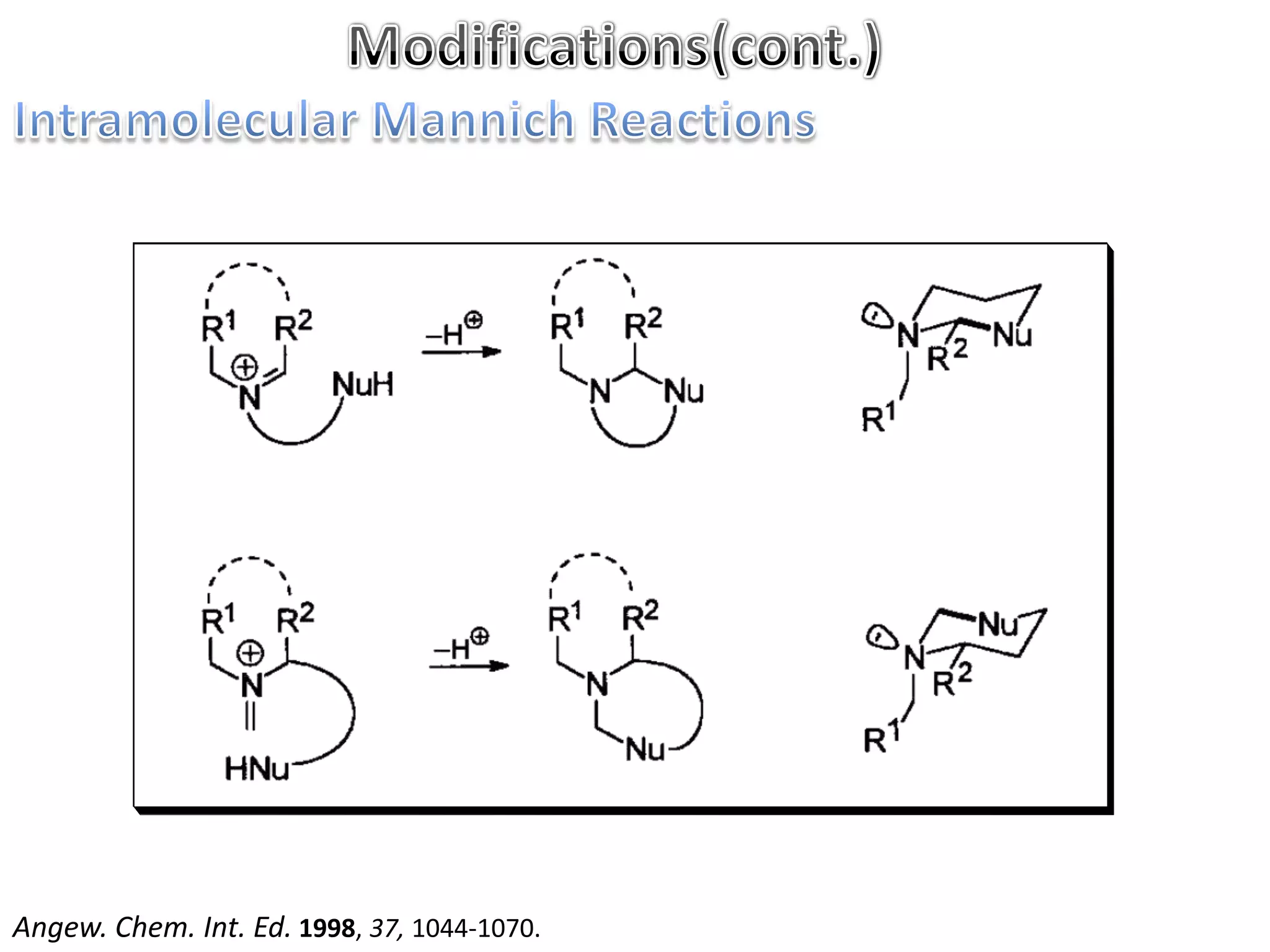
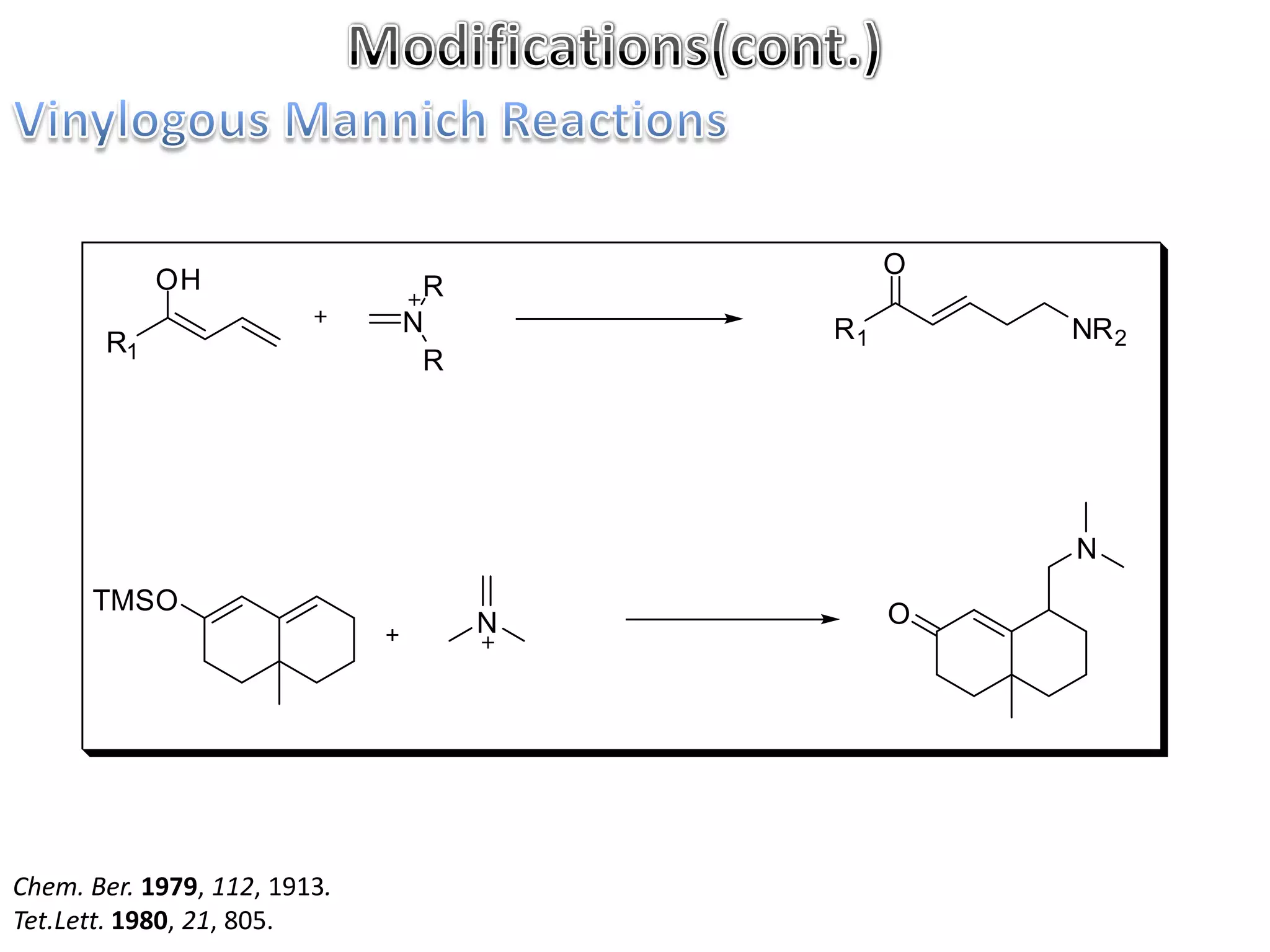
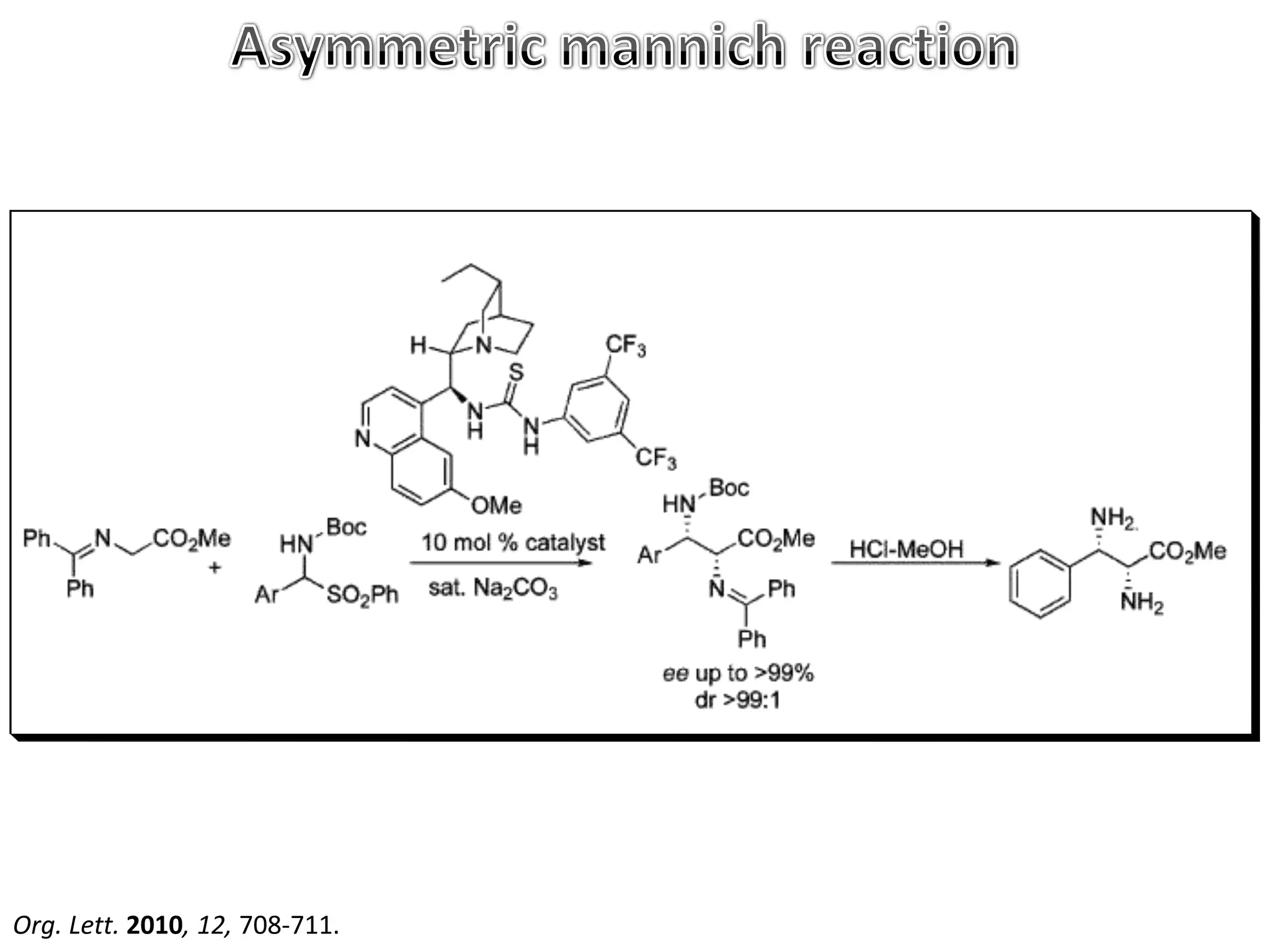
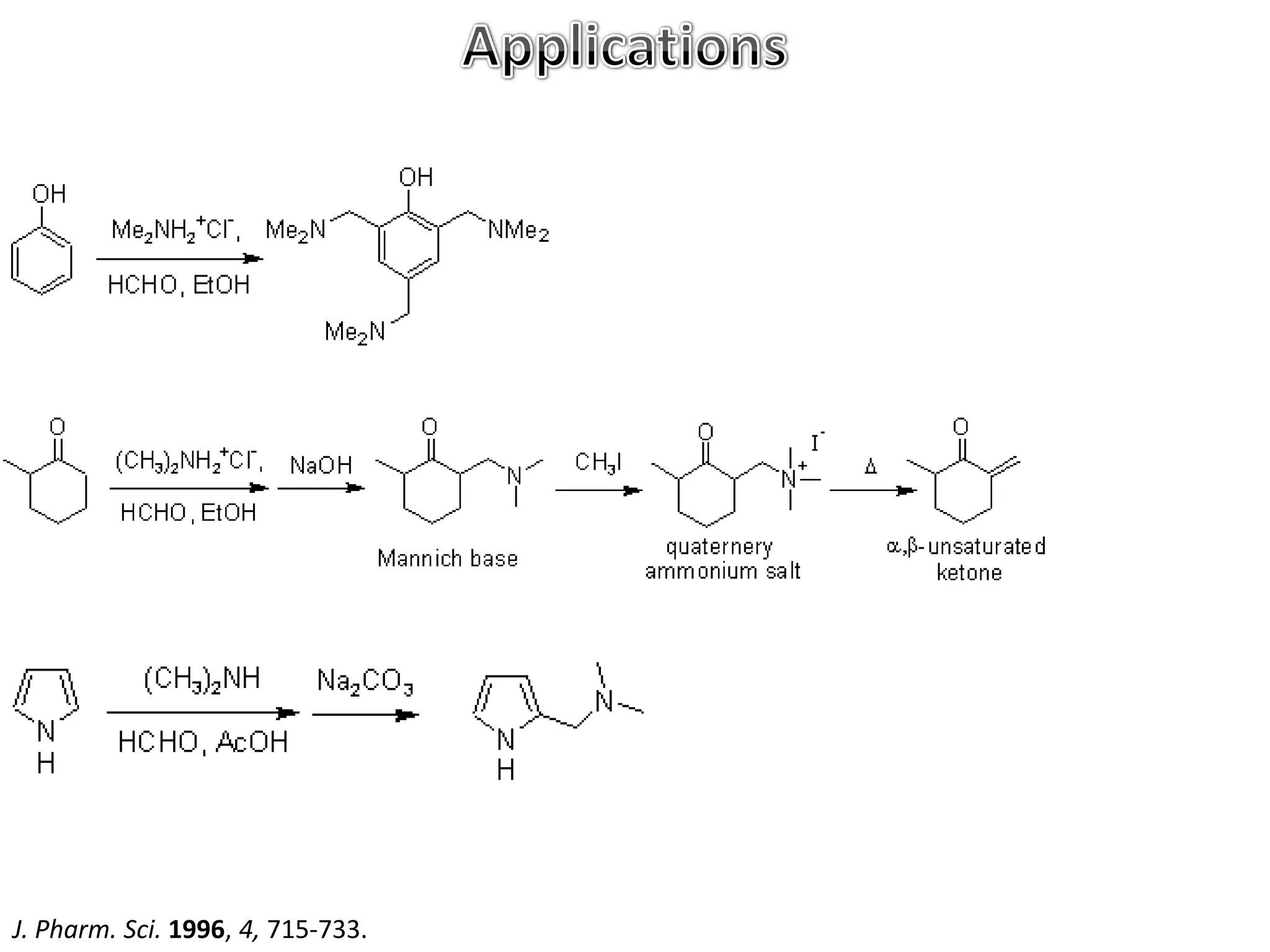
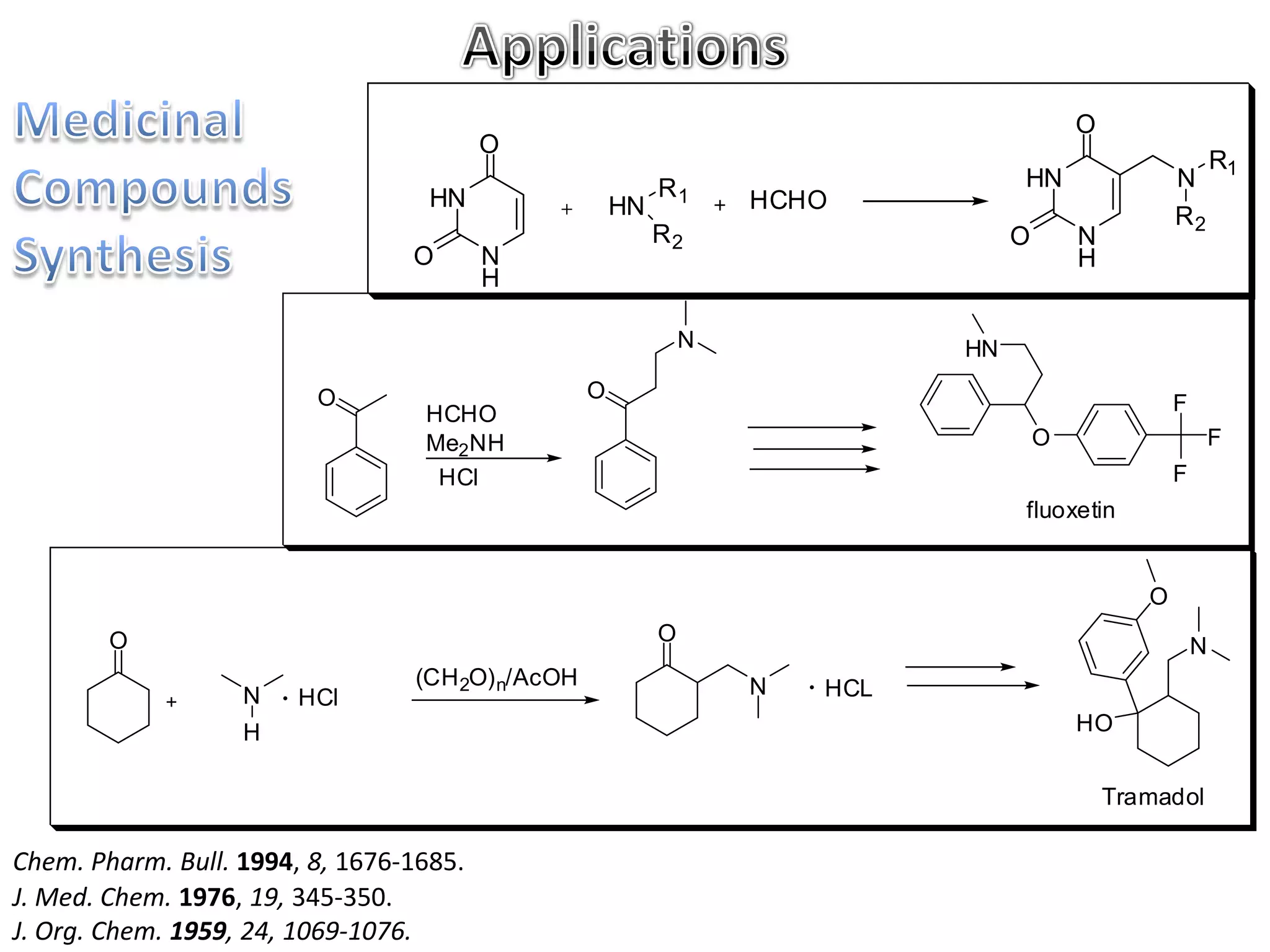
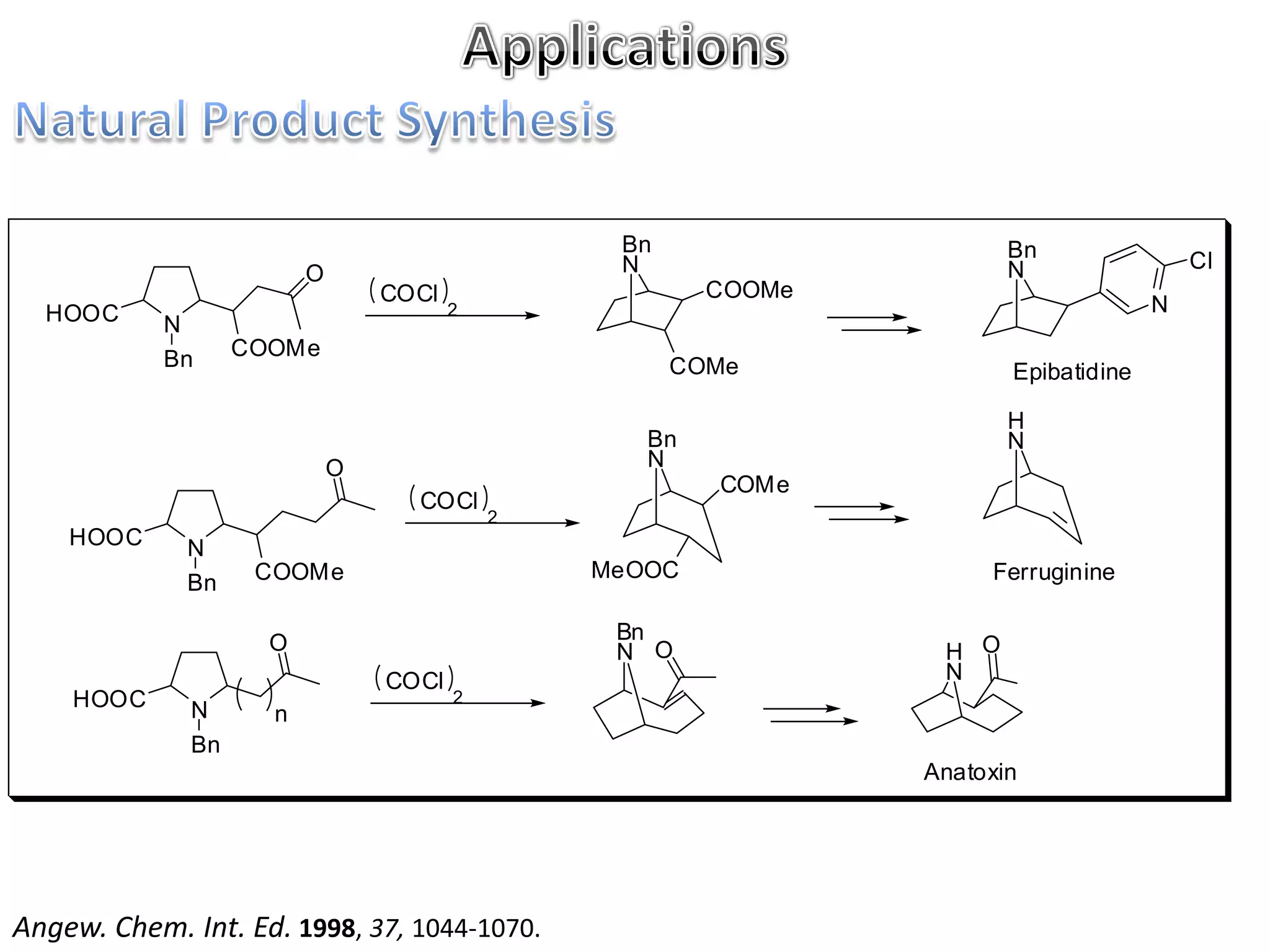
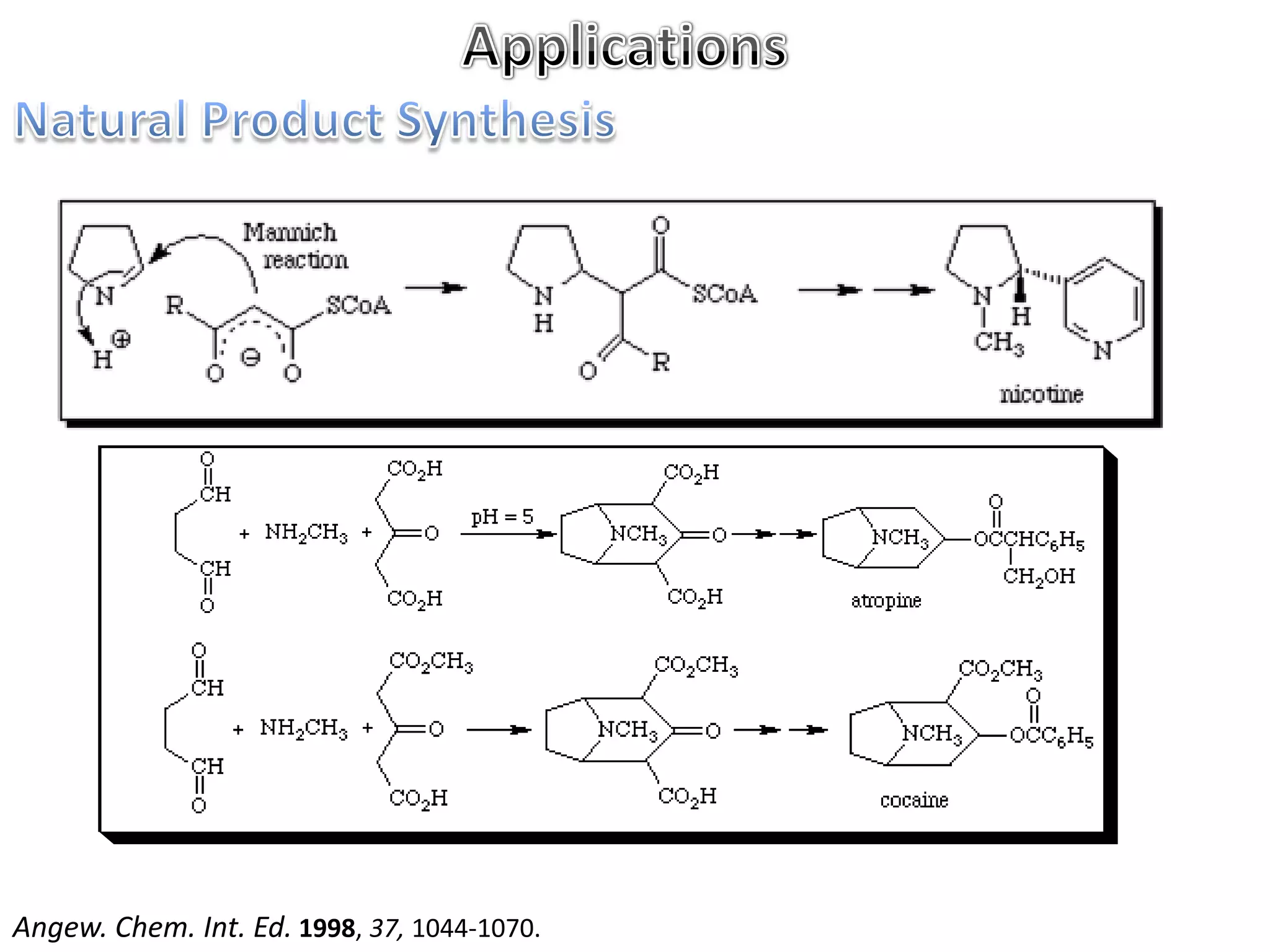
The Mannich reaction involves the condensation of an enolizable carbonyl compound, an aldehyde such as formaldehyde, and a primary or secondary amine. This results in an aminoalkylation and formation of a β-aminocarbonyl compound known as a Mannich base. Modifications using preformed Mannich bases and reactive substrates extend the scope and selectivity of the reaction. The Mannich reaction has wide applications in organic synthesis and for producing natural and medicinal compounds.