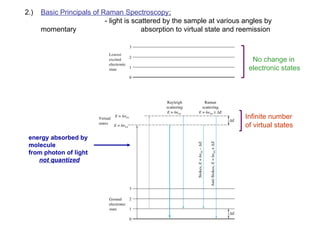
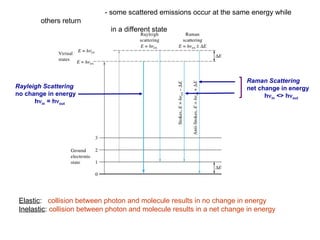
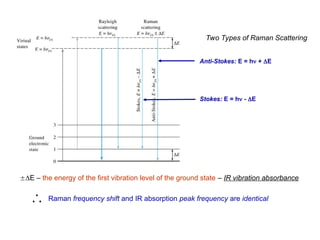
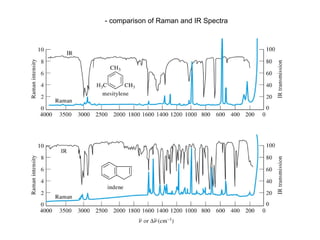
The document provides an overview of Raman spectroscopy, emphasizing its complementary relationship with infrared (IR) spectroscopy, and detailing its basic principles, instrumentation, and applications. It describes the mechanisms of Raman and IR, the significance of changes in polarizability for Raman activity, and the advantages of Raman over IR in certain contexts. Additionally, the document discusses qualitative and quantitative applications of Raman spectroscopy, including techniques like resonance Raman and surface-enhanced Raman spectroscopy.