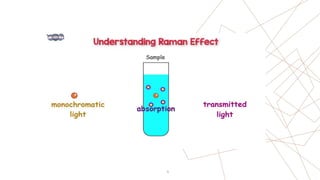
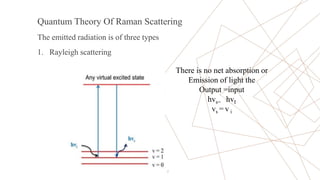
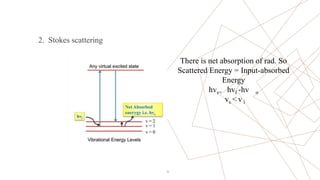
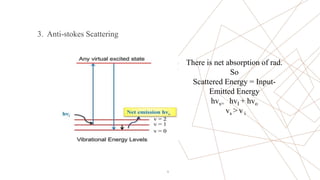
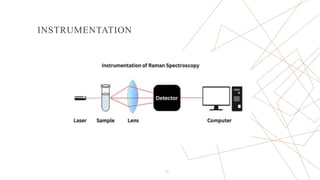
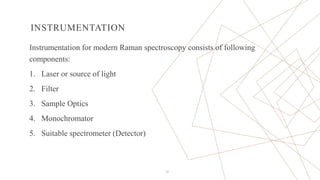
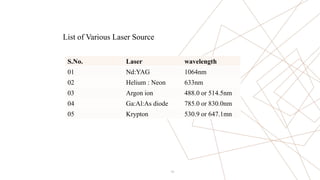
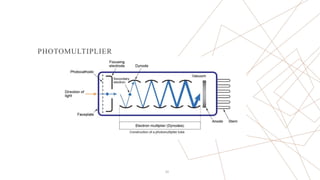
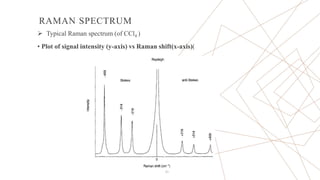
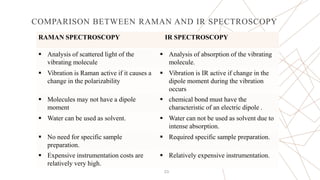
Raman spectroscopy is a spectroscopic technique used to observe vibrational modes of a system. It relies on inelastic scattering of monochromatic light, usually from a laser. When light interacts with molecules, the light may be scattered elastically (Rayleigh scattering) or inelastically (Raman scattering). A Raman spectrometer consists of a laser, filters, sample optics, monochromator, and detector. Raman spectra provide a fingerprint that can be used to identify molecules based on their vibrational energies. Compared to infrared spectroscopy, Raman spectroscopy can analyze solids, liquids, and gases and is not interfered by water. It has applications in chemistry, biology, and materials analysis.