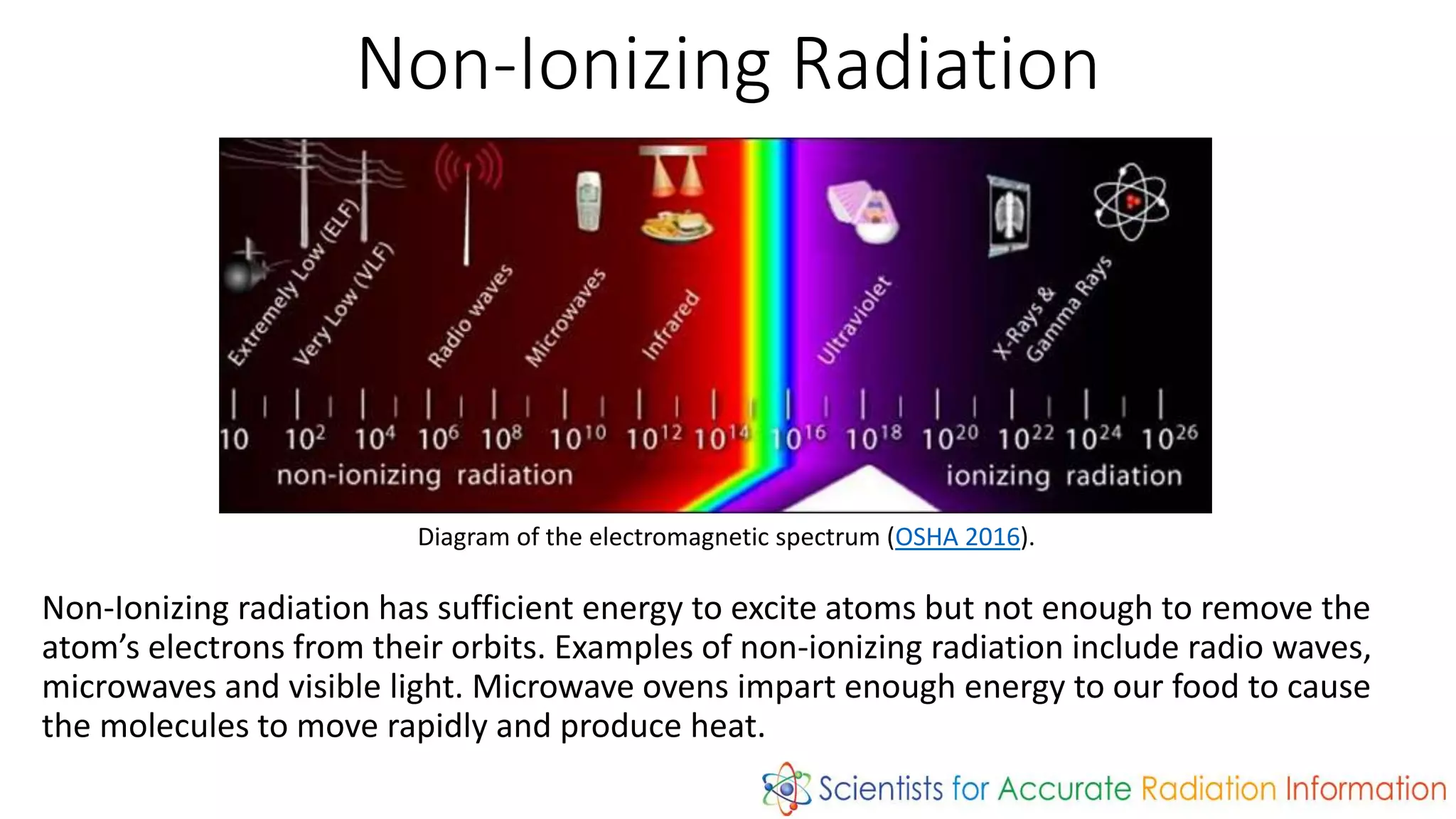
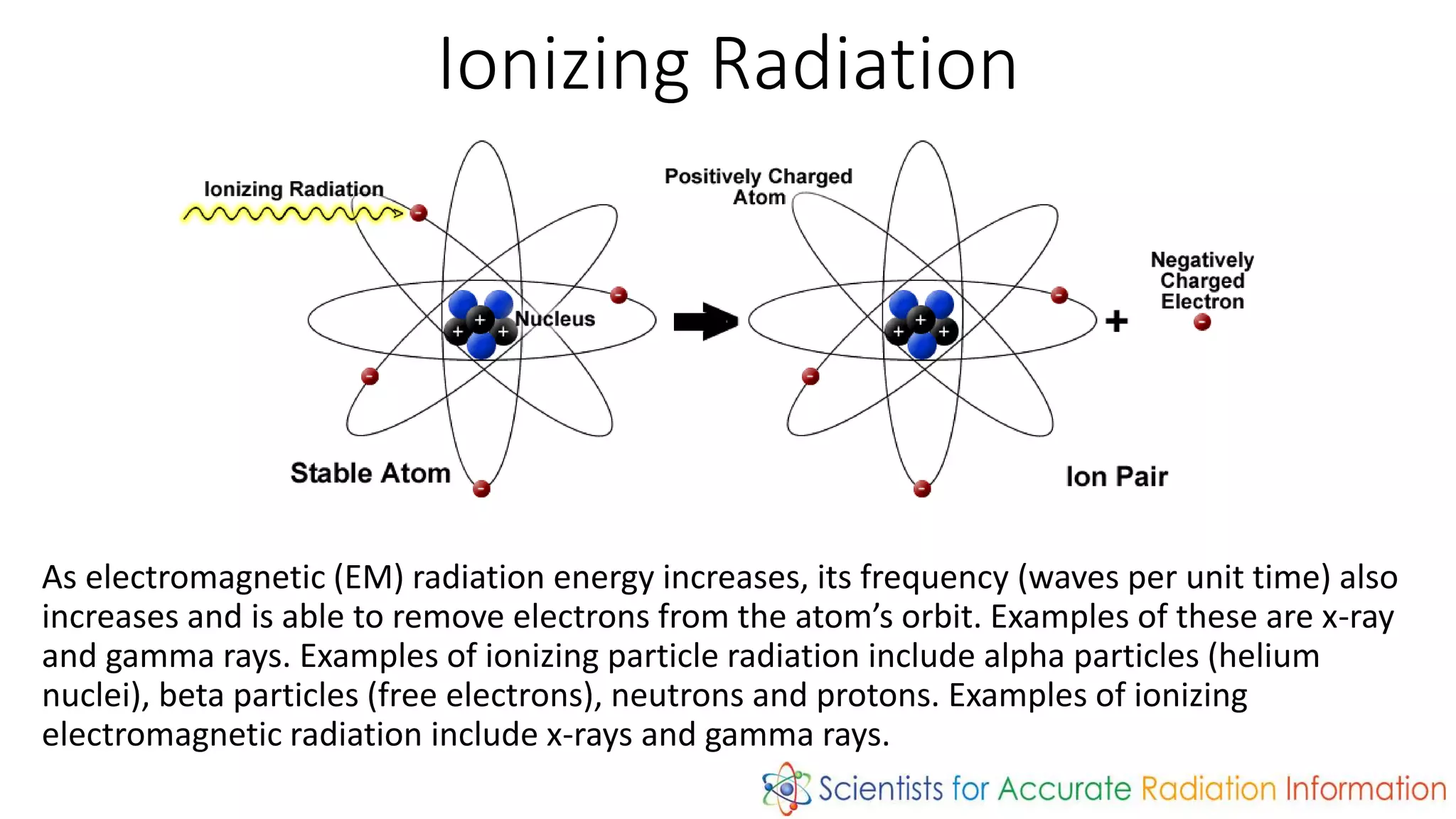
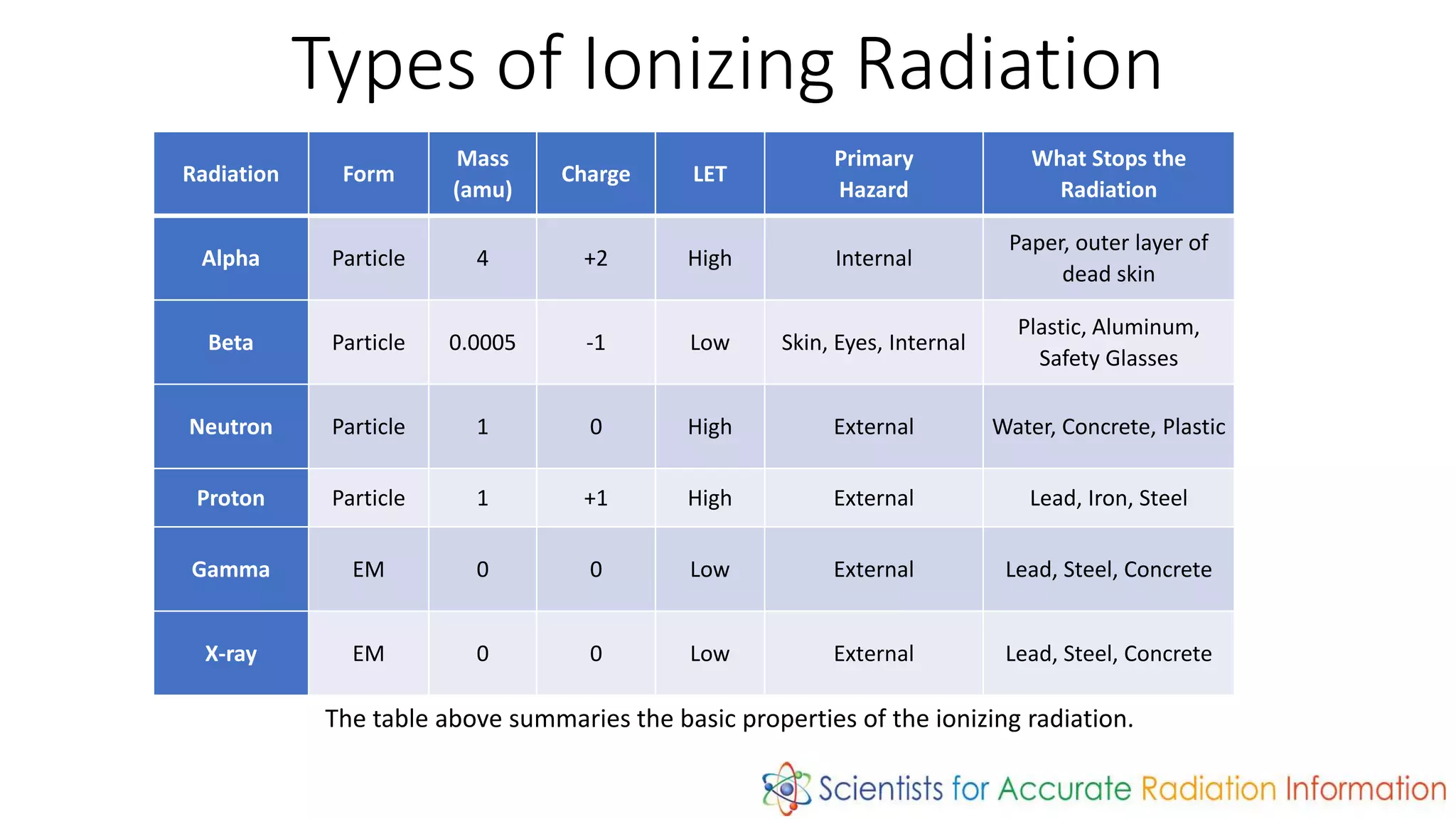
Radiation is energy emitted in the form of electromagnetic waves or particles. There are two types: non-ionizing radiation like radio waves which has enough energy to excite atoms but not remove electrons, and ionizing radiation like x-rays and gamma rays which can remove electrons. Ionizing radiation includes alpha particles, beta particles, neutrons, protons, x-rays and gamma rays. The amount of time for a radioactive substance to decay to half its original amount is called its half-life, which is useful in applications like radioactive dating and nuclear medicine.