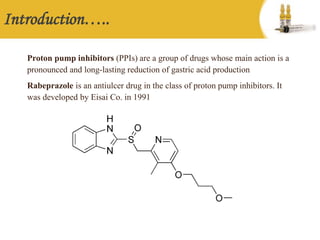
This document provides information on rabeprazole sodium for injection. It discusses the mechanism of action as a proton pump inhibitor that suppresses gastric acid production. It notes the pharmacokinetics of rabeprazole including its plasma half-life and metabolism. The recommended uses and dosing schedule of intravenous administration of 20 mg once daily are described. Potential side effects and precautions for use in pregnancy and breastfeeding are also outlined.