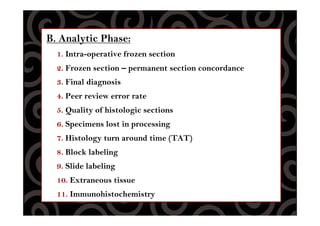
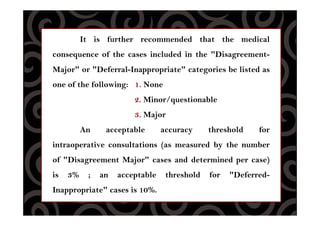
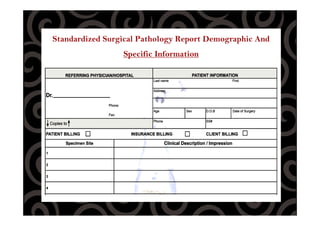
This document discusses quality control procedures for surgical pathology services. It defines key terms like quality assurance, quality control, and quality improvement. It then outlines the phases of quality control including pre-analytic, analytic, and post-analytic phases. The document provides details on approaches to quality control, including monitoring specimen handling and processing, diagnostic accuracy and turnaround times, pathology reporting standards, and ensuring diagnostic findings are integrated with ancillary study results.