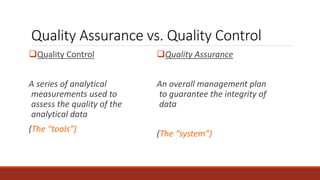
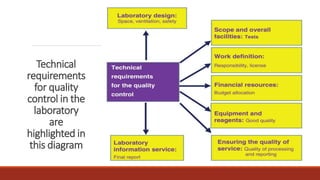
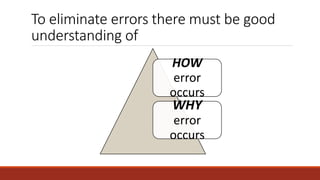
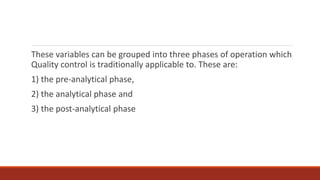
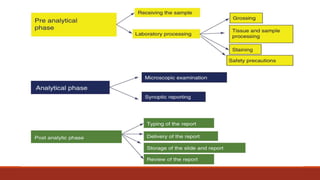
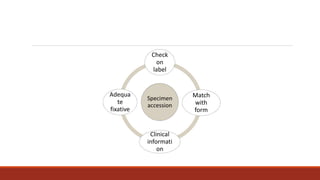
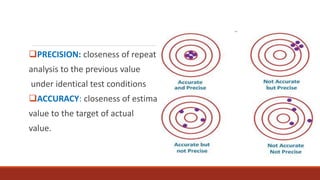
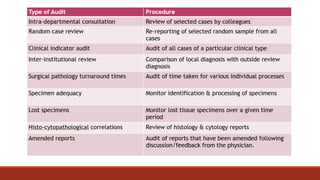
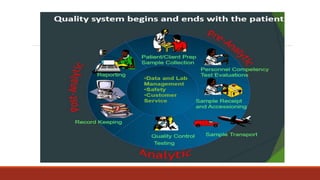
This document discusses quality control in histopathology. It defines key terms like quality control, quality assurance, and total quality management. It outlines the pre-analytical, analytical and post-analytical phases of quality control and highlights variables that can affect quality in each phase like personnel training, equipment, and interpretation of results. It provides recommendations for achieving quality control through standardized procedures, monitoring turnaround times, participation in proficiency testing, and review of reports. The goal is to generate accurate histopathology reports and enable easy retrieval if needed.