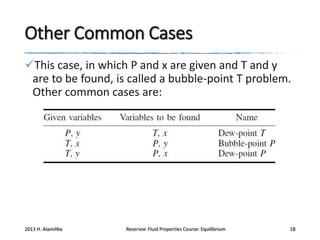
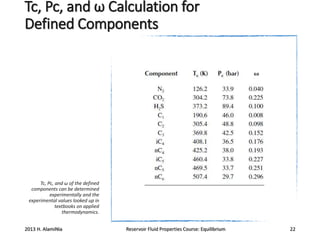
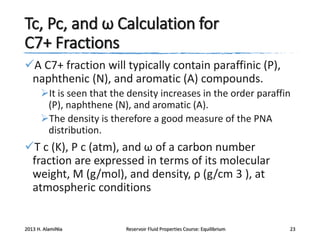
This document provides an overview of key concepts for performing phase equilibrium calculations on reservoir fluids, including:
1) Cubic equations of state and properties required for components in mixtures like critical temperature, pressure, and acentric factor.
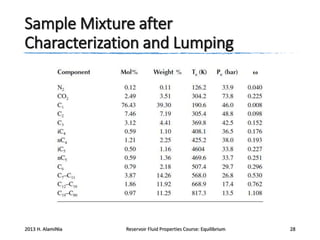
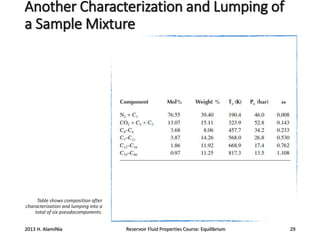
2) Calculating these properties for hydrocarbon components and lumping heavier fractions into pseudocomponents.
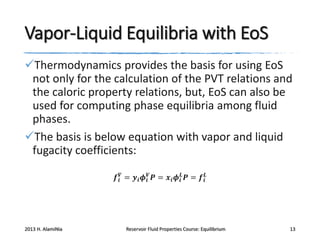
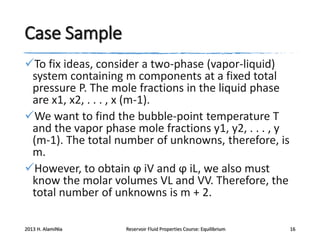
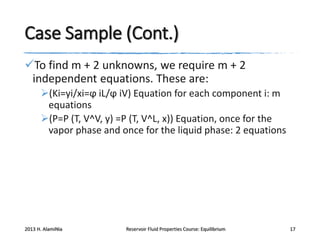
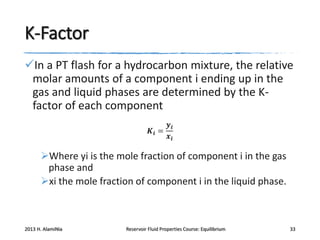
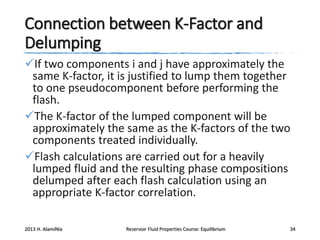
3) Using equations of state to relate fugacity coefficients to vapor-liquid equilibrium and calculate K-factors for flash calculations.