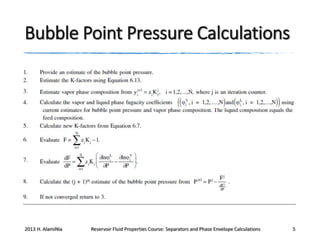
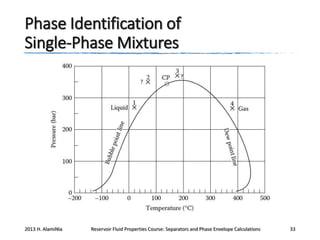
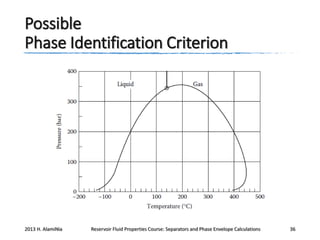
This document outlines course material on reservoir fluid properties, separators, and phase envelope calculations. It covers topics such as PT flash processes, mixture saturation points, phase envelope determination using Michelsen's technique, and separator calculations to optimize pressure and determine stock tank oil properties. Examples of phase envelopes are shown for oil and gas condensate mixtures, illustrating properties like critical points. The document provides information to understand fluid behavior relevant to production operations.