The document discusses H2 antagonists, a class of drugs that block histamine action at H2 receptors to reduce gastric acid secretion, thus aiding in the treatment of peptic ulcers and related conditions. It covers the mechanisms of action, structure-activity relationships, pharmacokinetics, adverse effects, and therapeutic uses of specific H2 antagonists like cimetidine, famotidine, and ranitidine, along with proton pump inhibitors. References for further reading on medicinal chemistry are also provided.





![H2 ANTAGONIST
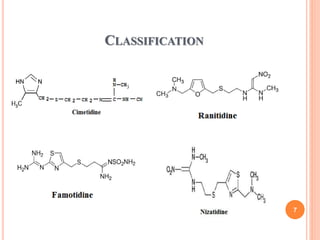
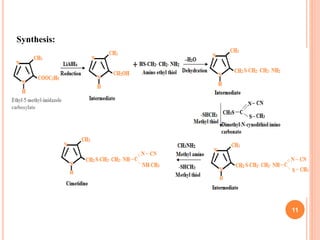
Cimetidine
Structure:
IUPAC: 1-Cyano-3-methyl-2-(2-{[(5-methyl-1H-imidazole) methyl]
sulfanyl}ethyl)guanidine
Properties:
White or off white crystalline powder, unpleasant odor, sparingly
soluble in water but soluble in alcohol
10
Molecular structure:C10H16N6S](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-10-320.jpg)


![ Famotidine
Structure:
IUPAC:3-[({2-[(diaminomethylidene)amino]-1,3-thiazole}methyl)
sulfanyl]-N'-sulfamoylpropanimidamide
Properties:
White to pale yellow crystals, freely soluble in glacial acetic acid,
slightly soluble in methanol, very slightly soluble in water, and
practically insoluble in ethanol.
14
Molecular formula:C8H15N7O2S3](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-14-320.jpg)

![Ranitidine
Structure:
IUPAC:[1-({2-[({5-[(dimethylamino)methyl]furan}methyl)sulfanyl]
ethyl}amino)-2-nitroethenyl](methyl)amine
Properties:
white to pale yellow, granular substance, Characteristic odour, Bitter
taste, Water soluble, sensitive to light and moisture.
16
Molecular formula: C13H23N4O3S](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-16-320.jpg)

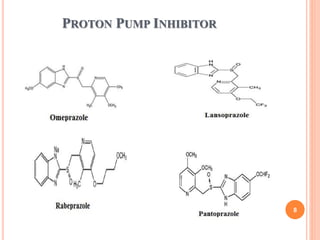
![Omeprazole
Structure:
IUPAC:6-methoxy-2-[(4-methoxy-3,5-
dimethylpyridine)methanesulfinyl]-1H-1,3-benzodiazole
Properties:
white hygroscopic powder, soluble in water, alcohol, propylene
glycol, very slightly soluble in methylene chloride. 20
Molecular formula: C17H19N3O3S](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-20-320.jpg)


![Pantoprazole
Structure:
IUPAC:6-(difluoromethoxy)-2-[(3,4-
dimethoxypyridine)methanesulfinyl]-1H-1,3-benzodiazole
Properties:
White color, soluble in water, insoluble in hexane, slightly soluble in
buffer pH 7.4.
23
Molecular formula:C16H14F2N3O4S](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-23-320.jpg)


![Lansoprazole
Structure:
IUPAC:2-{[3-methyl-4-(2,2,2-trifluoroethoxy)
pyridine]methanesulfinyl}-1H-1,3-benzodiazole
Properties:
White to Brownish white crystalline powder, odorless, freely soluble
in dimethyl formamide, soluble in methanol, slightly soluble in ethyl
acetate, acetonitrile, very slightly soluble in water, heaxanes 26
Molecular formula:C16H13F3N3NaO2S](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-26-320.jpg)


![Rabeprazole
Structure:
IUPAC:2-{[4-(3-methoxypropoxy)-3-methylpyridine]
methanesulfinyl}-1H-1,3-benzodiazole
Properties:
White to yellow solid, soluble in water, methanol, freely soluble in
ethanol, chloroform, ethylacetate, insoluble in ether. 29
Molecular formual:C18H21N3O3S](https://image.slidesharecdn.com/1stunit-h2antagonist-200926135648/85/1-st-unit-h2-antagonist-29-320.jpg)