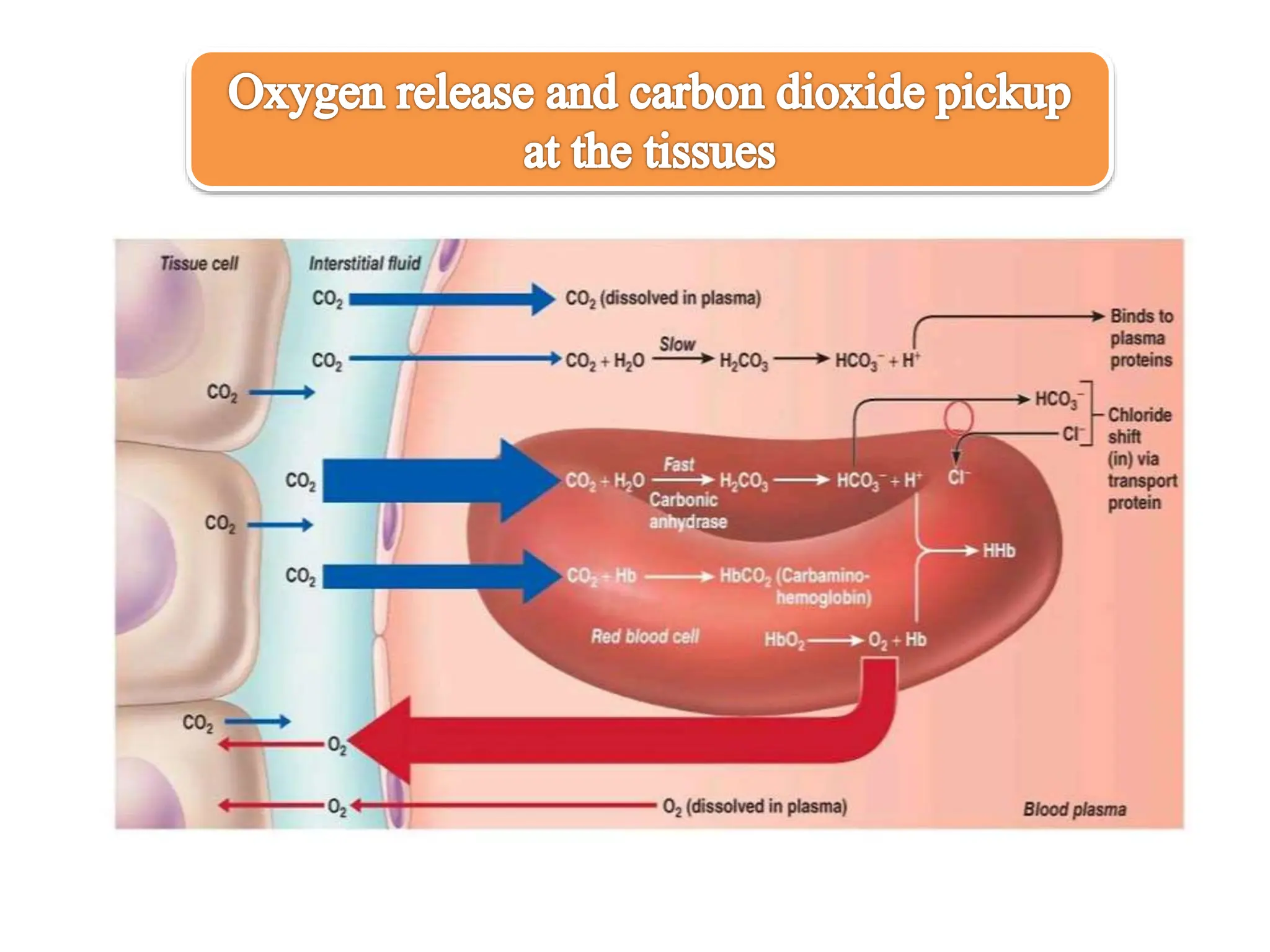
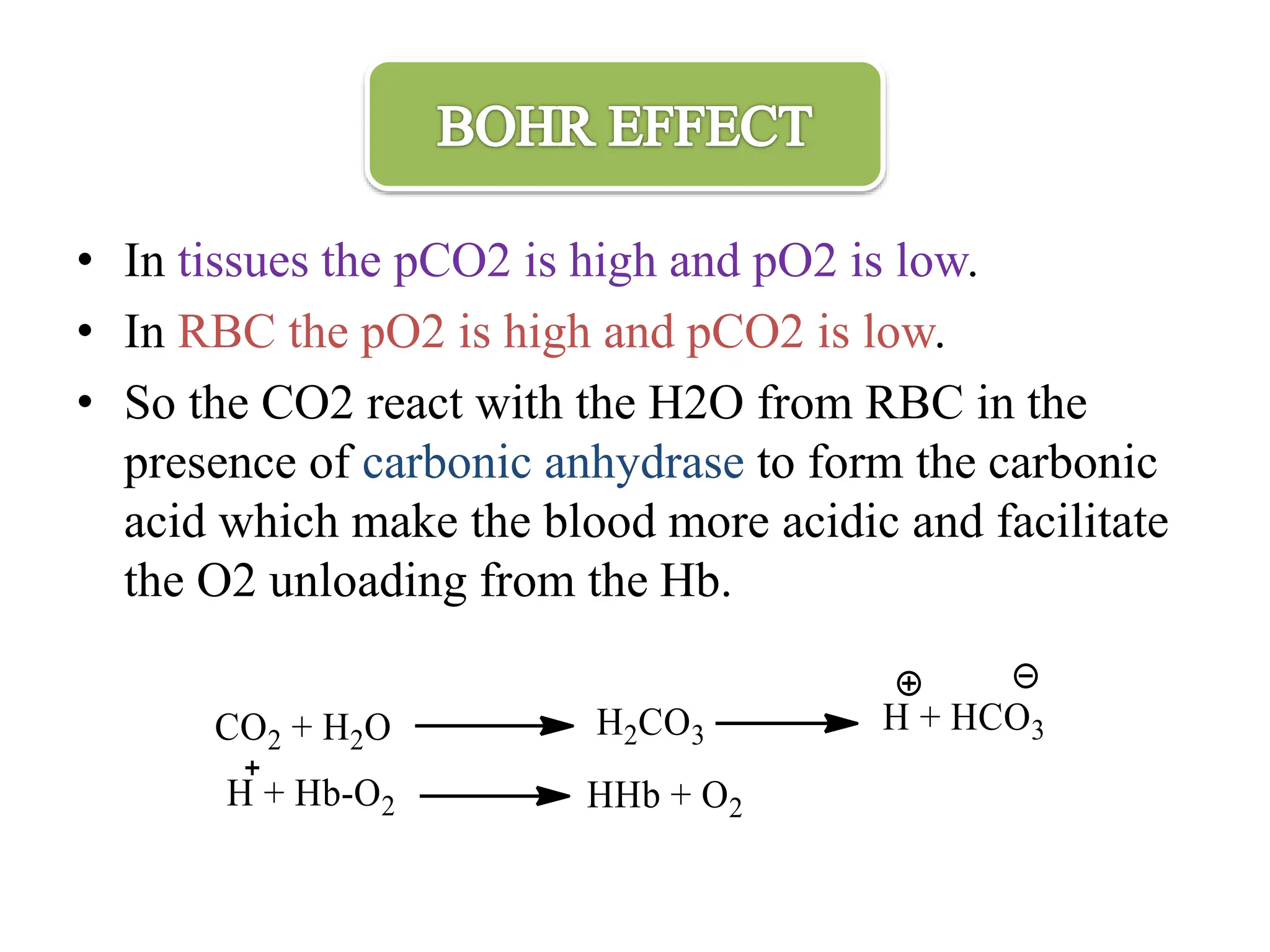
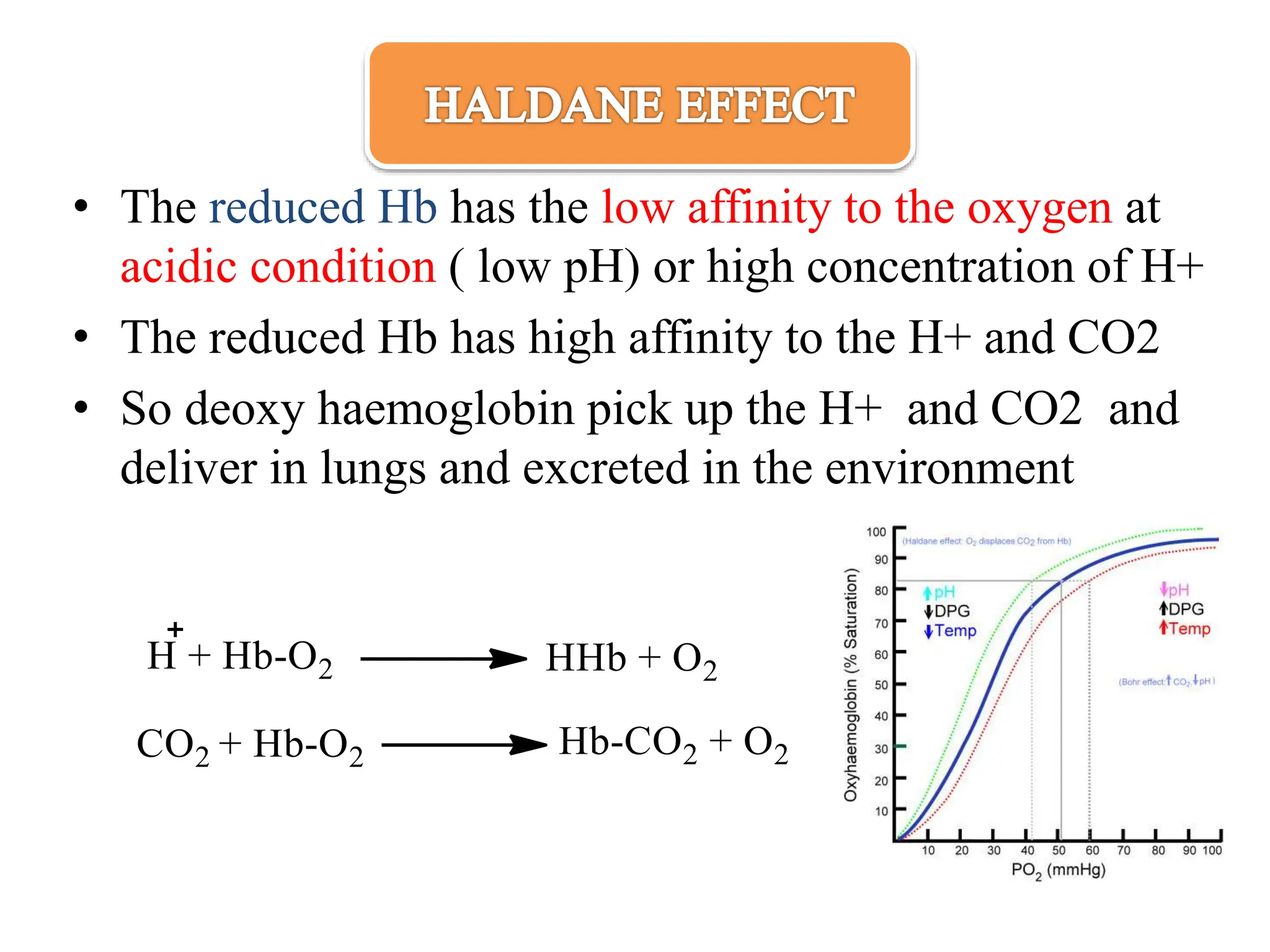
The document discusses the properties and functions of hemoglobin (Hb) regarding oxygen and carbon dioxide transport in the blood. It explains the Bohr and Haldane effects, which describe how pH and CO2 concentration influence Hb's affinity for oxygen and its ability to carry CO2. Additionally, it covers the structural changes in hemoglobin during oxygen binding, the role of 2,3-bisphosphoglycerate in oxygen release, and the differences between adult and fetal hemoglobin affinities.