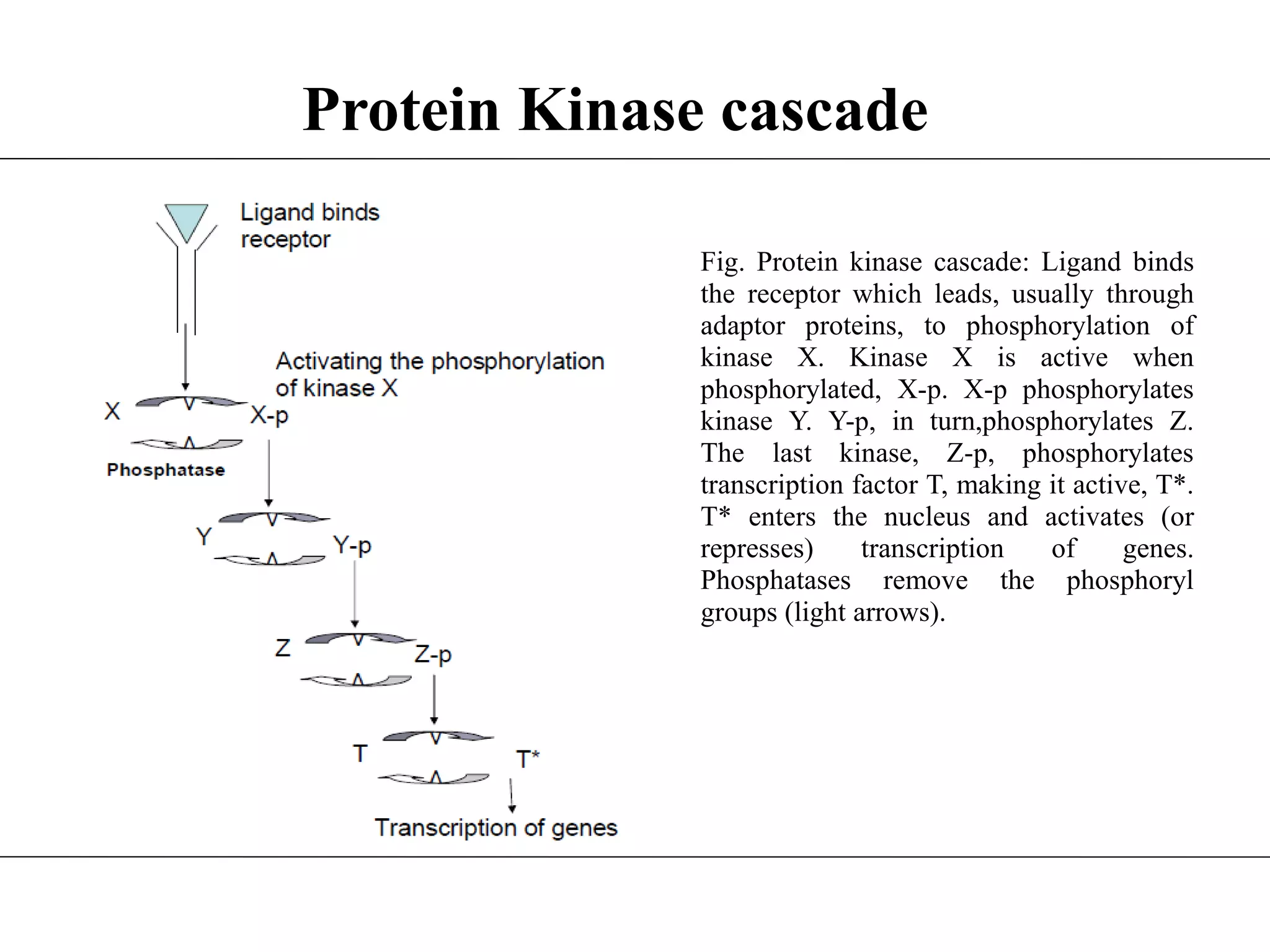
This document provides an overview of protein kinase cascades. It begins with an introduction to protein kinases and their function in phosphorylating proteins and playing roles in cellular processes. It then discusses the classification of protein kinases based on the amino acid they phosphorylate. Next, it explains the basic mechanism of phosphorylation by protein kinases using ATP. It proceeds to describe protein kinase cascades where one kinase phosphorylates and activates the next in a chain. Examples of double phosphorylation and multi-layer perceptrons in cascades are illustrated. Finally, some biological examples of different types of protein kinases are mentioned before concluding with references.