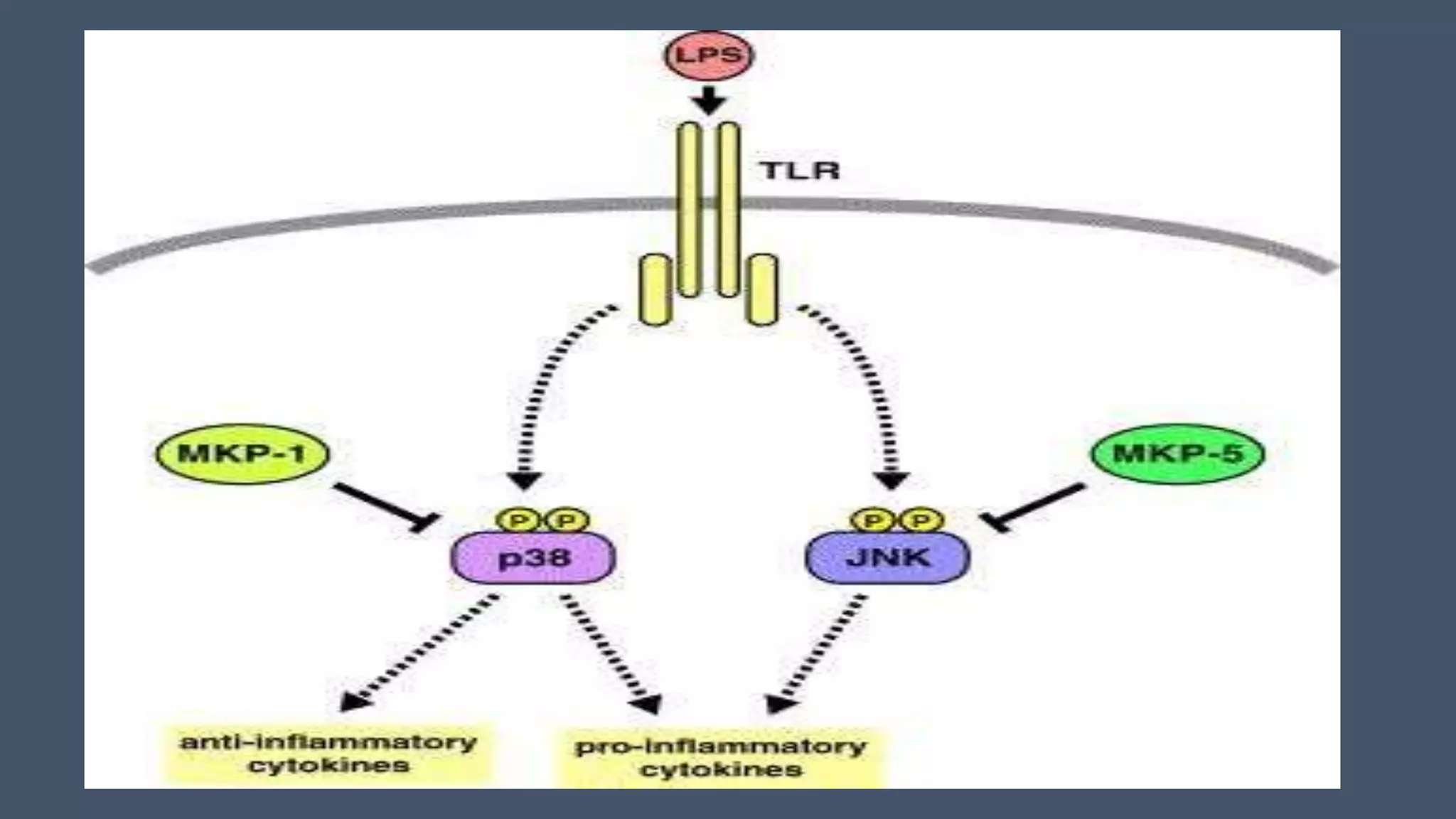
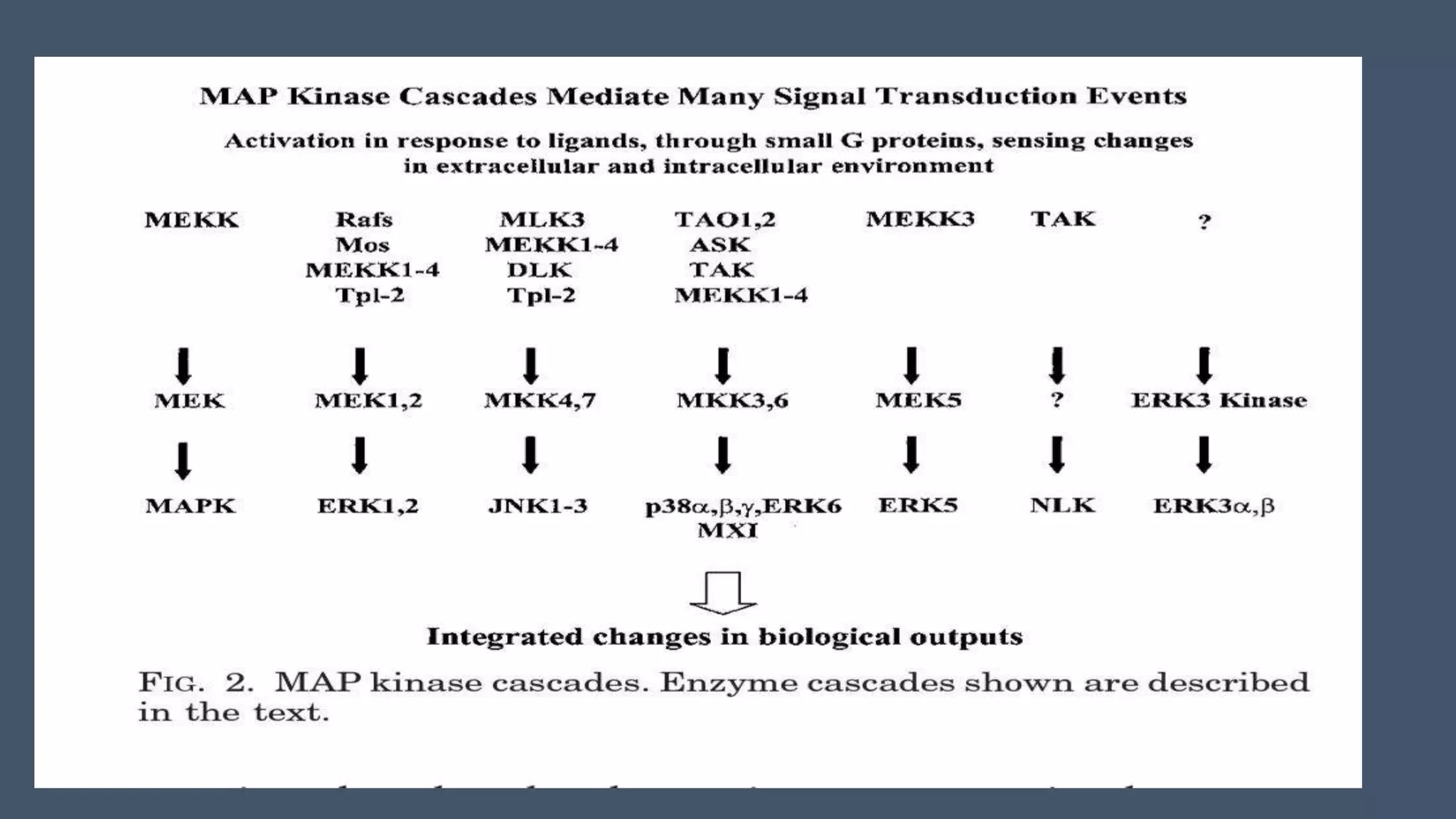
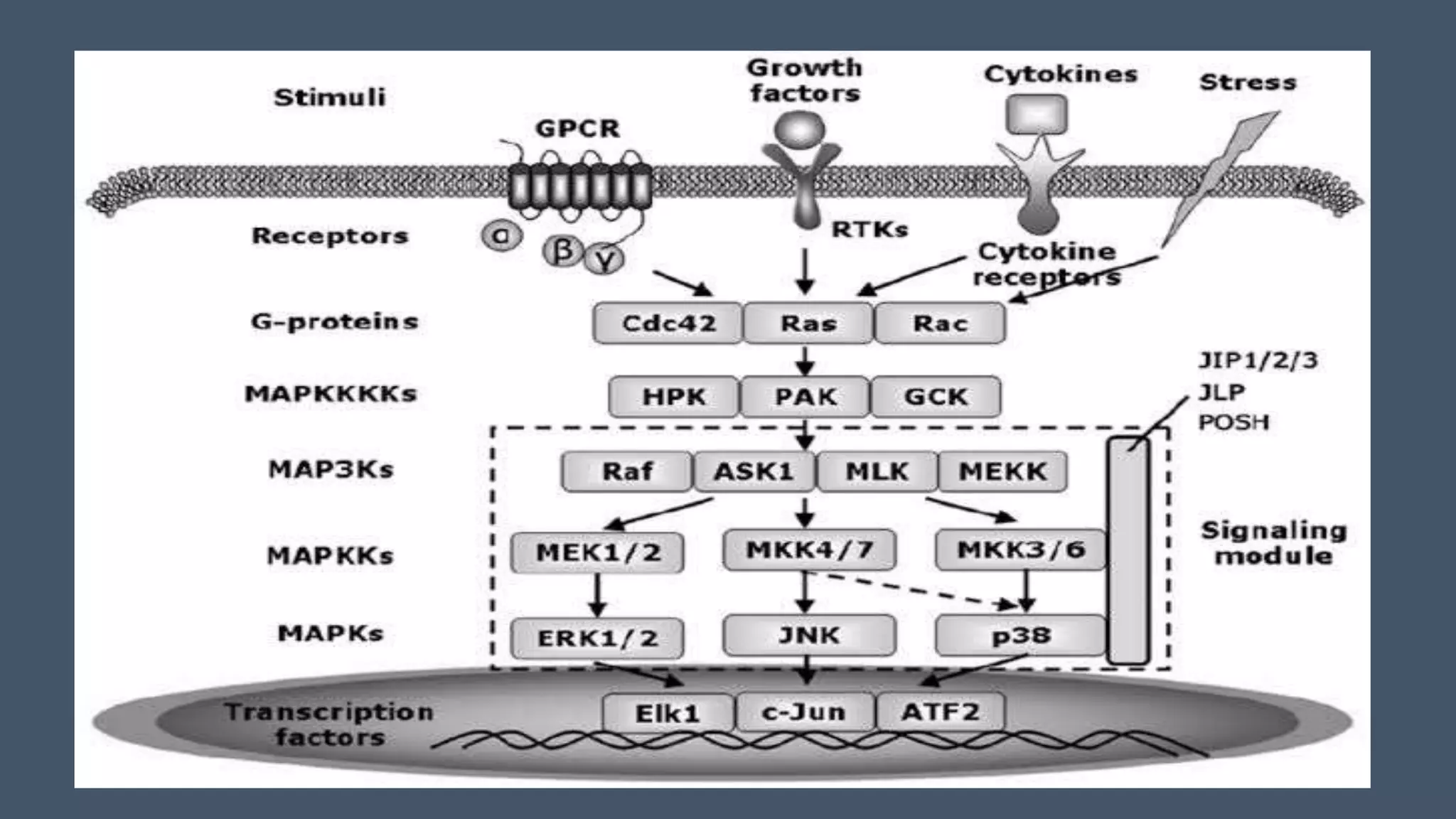
The document discusses the role of mitogen-activated protein kinases (MAPKs) in cellular responses to environmental changes, emphasizing their regulatory functions in processes like migration, proliferation, and differentiation. It describes the activation mechanisms of MAPKs through phosphorylation cascades and their interactions with other signaling pathways in both yeast and mammals. The review also outlines the historical context of MAPK research, highlighting specific pathways and their implications in various cellular functions.