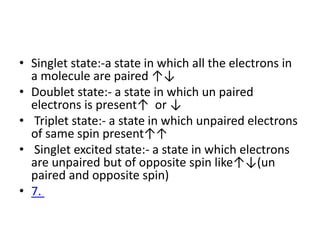
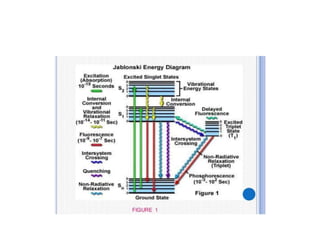
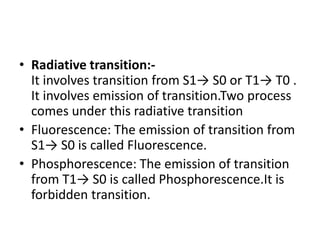
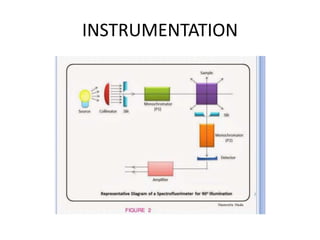
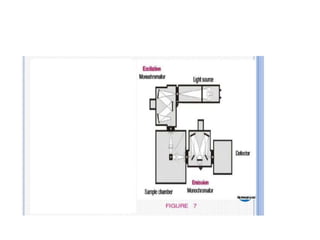
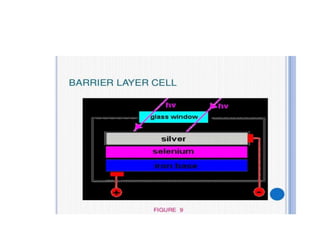
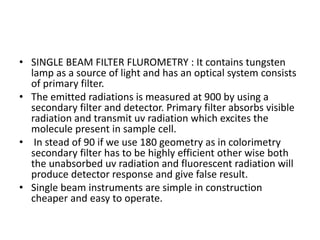
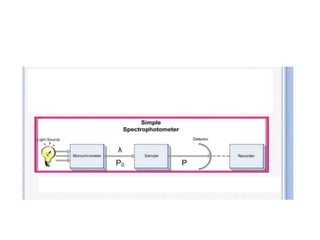
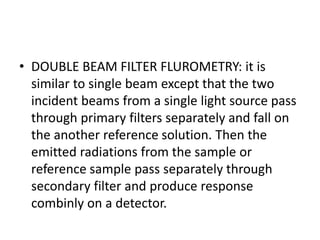
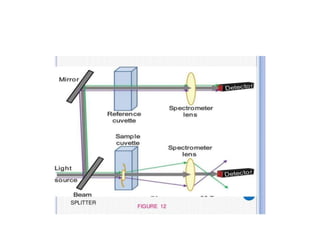
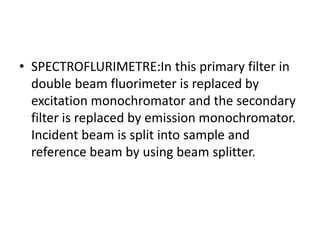
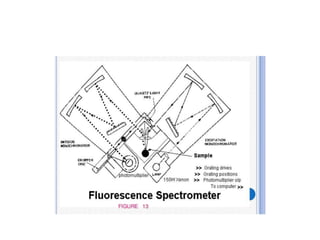
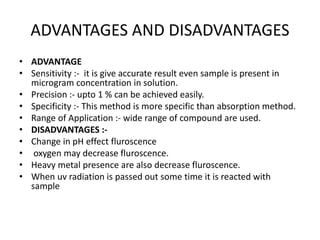
Fluorimetry is a technique that measures fluorescence intensity at a particular wavelength using a fluorimeter or spectrofluorimeter. It works by exciting a molecule's electrons with radiation, causing them to emit radiation upon returning to the ground state. Factors like concentration, quantum yield, incident light intensity, pH, temperature and presence of quenchers can affect fluorescence. Fluorimetry has advantages like high sensitivity, precision and specificity and is useful for determining various inorganic/organic substances and compounds.