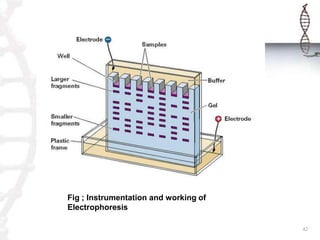
This document provides an overview of classical and bioanalytical methods for analyzing biomolecules. It discusses several classical methods like IR spectroscopy, UV-Vis spectroscopy, mass spectrometry, chromatography techniques and X-ray crystallography. It notes limitations of classical methods for complex biomolecules. The document then introduces bioanalytical methods like electrophoresis, ligand binding assays for improved analysis of proteins, DNA and other biomacromolecules. It provides details on the principles and procedures of electrophoresis as a key bioanalytical technique.



























![The electron density equation
1
(xyz) F(hkl) exp[ 2 i(hx ky lz) i hkl ]
V h k l
• h,k,l – indices of reflections
• xyz – coordinates
• F – amplitude of reflections
• – phase of reflections
• V- unit cell volume
32](https://image.slidesharecdn.com/presentation21-130226081033-phpapp01/85/Presentation2-1-32-320.jpg)