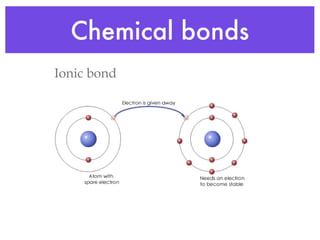
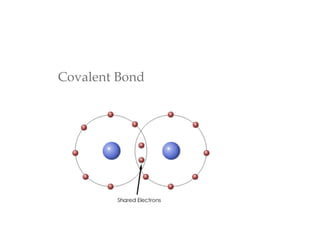
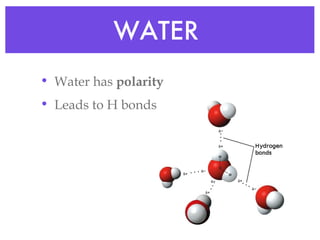
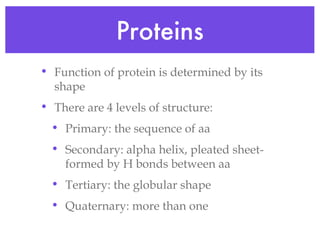
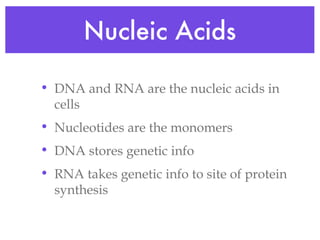
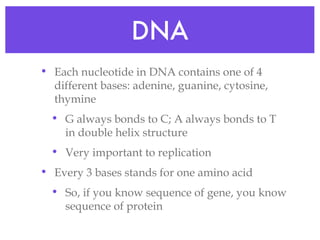
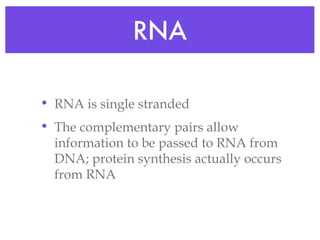
This document provides an overview of basic chemistry concepts and biological molecules important for cellular structure and function. It discusses the atomic nature of matter and how atoms bond via ionic and covalent bonds. The four main biomolecules - carbohydrates, lipids, proteins, and nucleic acids - are introduced along with their monomers, polymers, and roles. Key cellular structures like the plasma membrane and differences between prokaryotic and eukaryotic cells are also summarized.