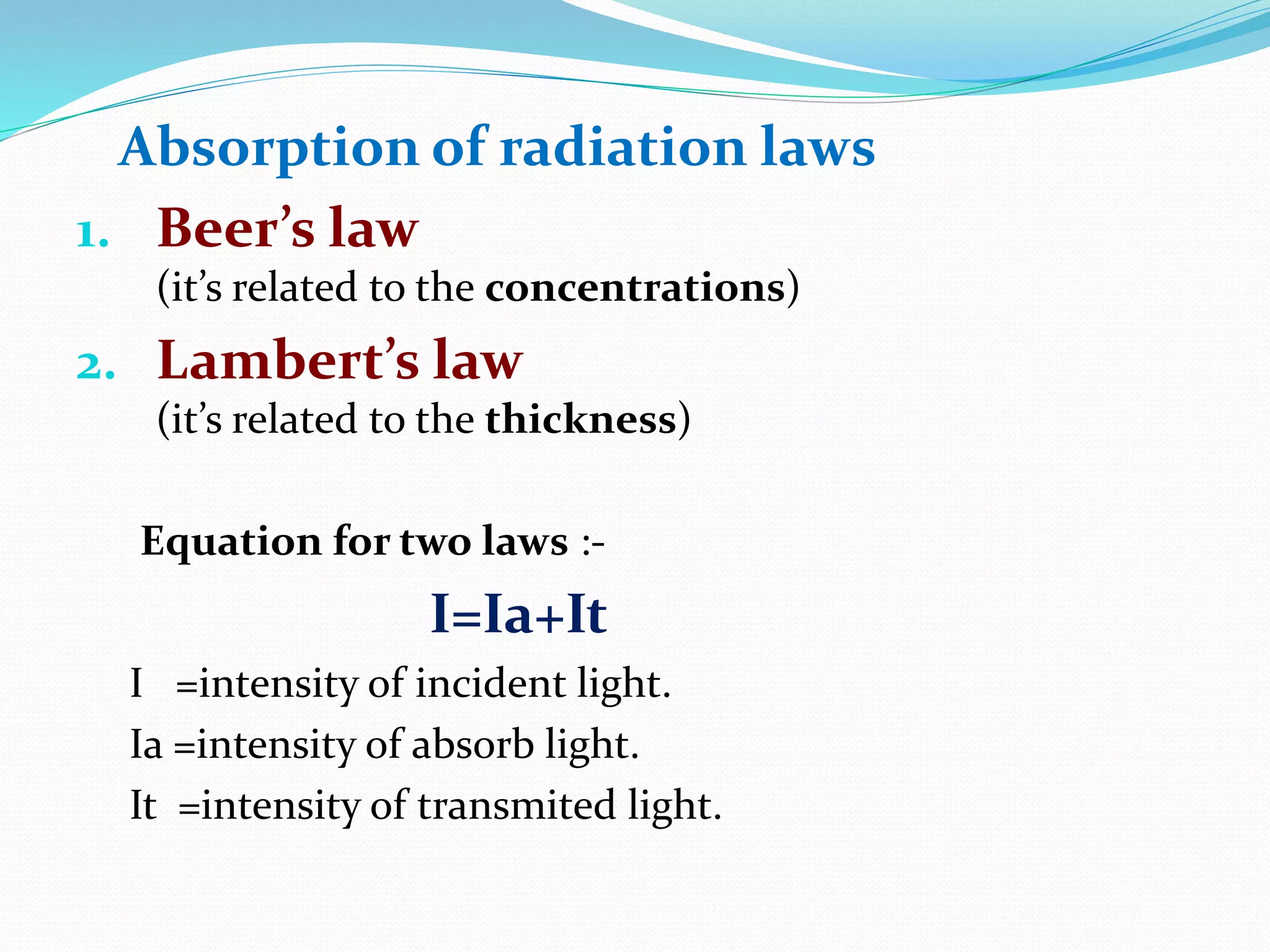
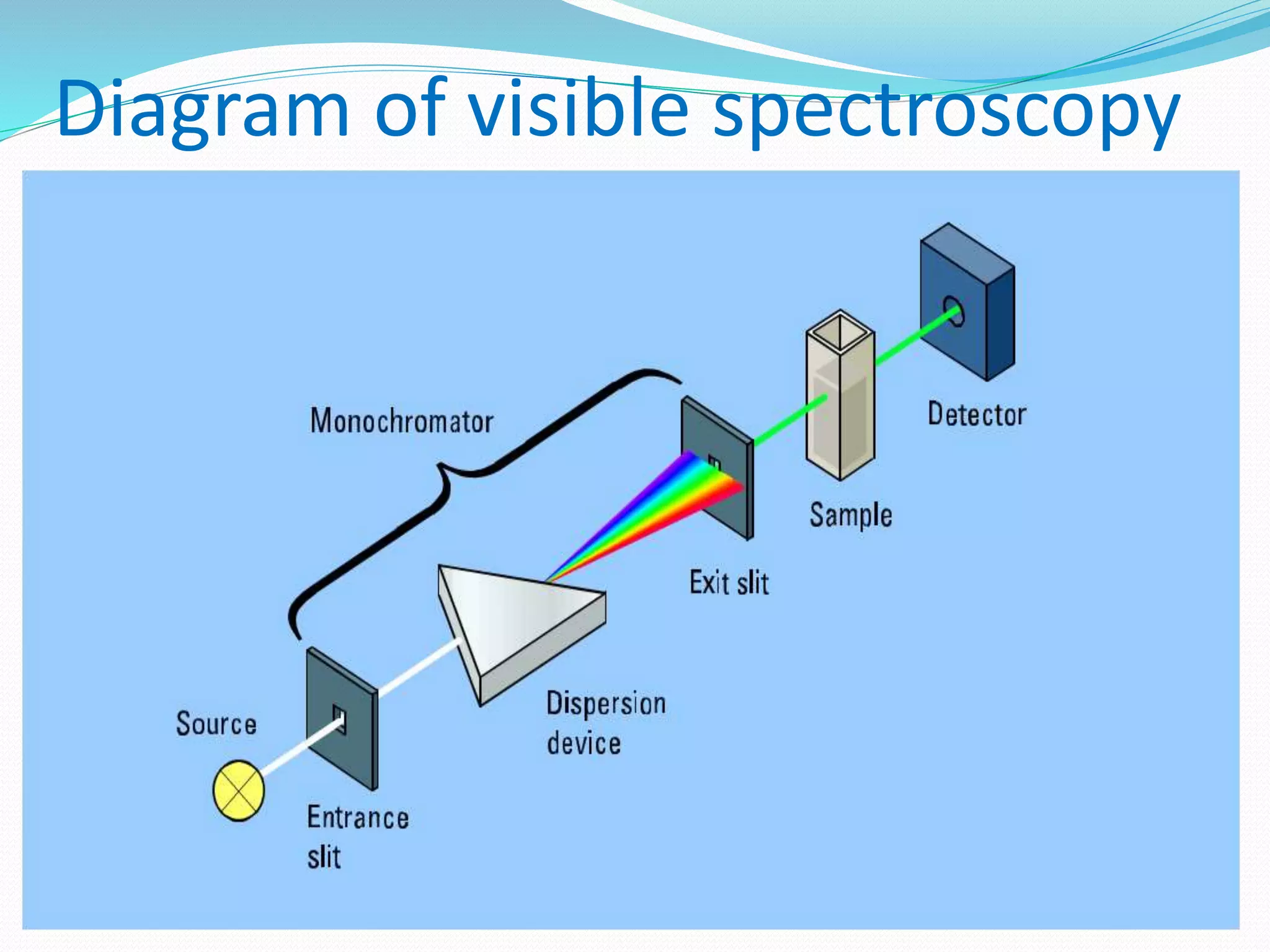
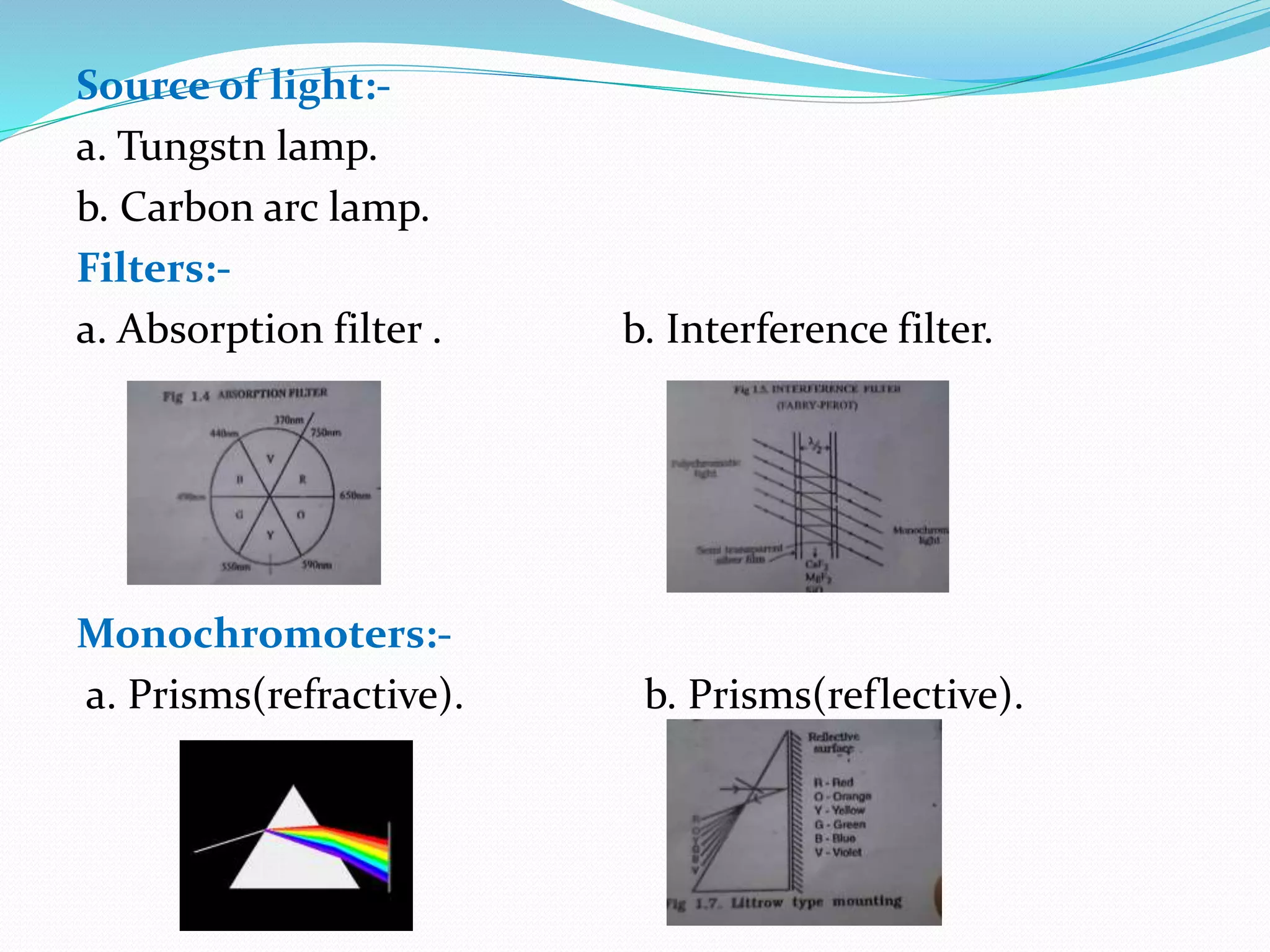
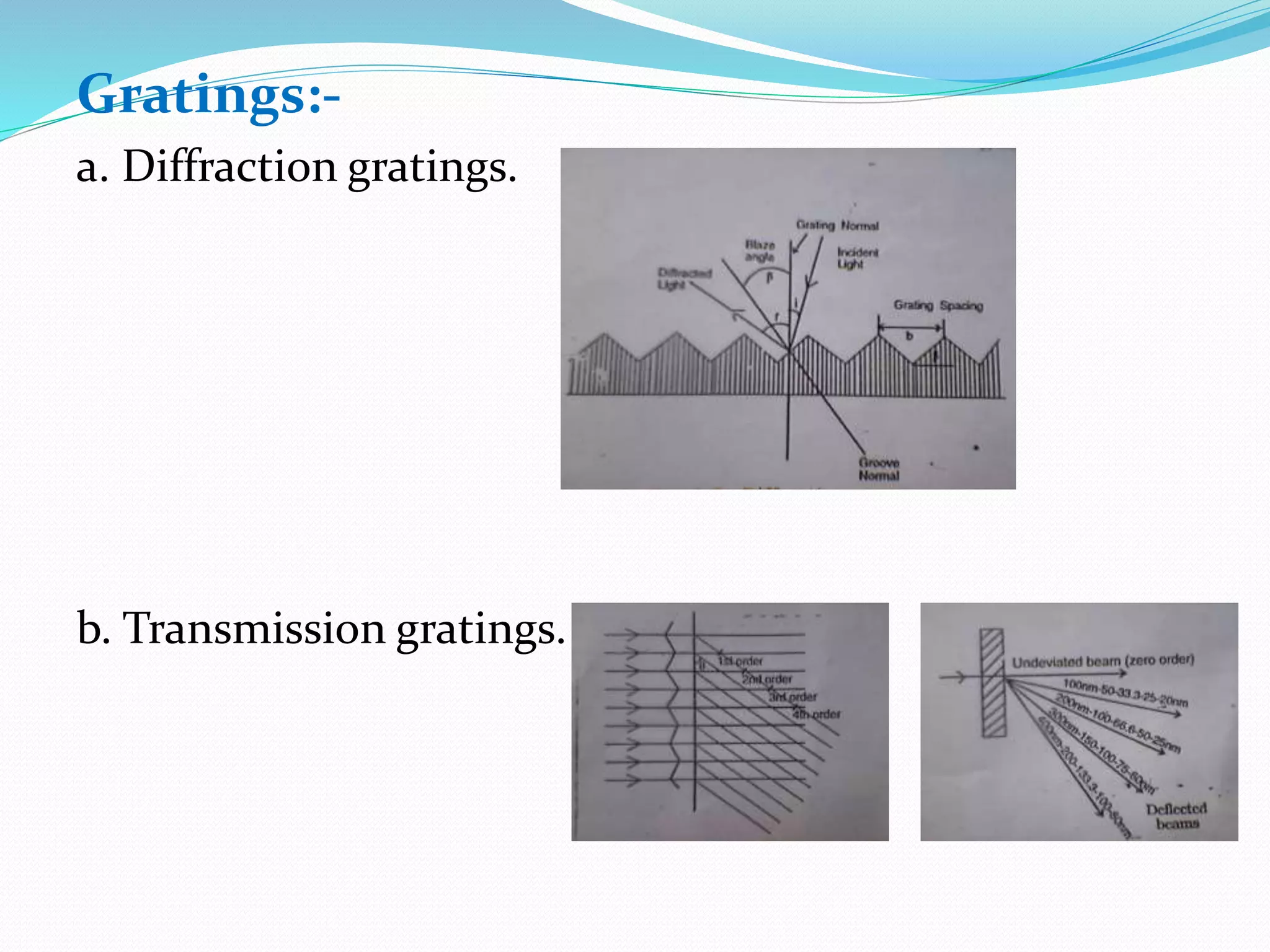
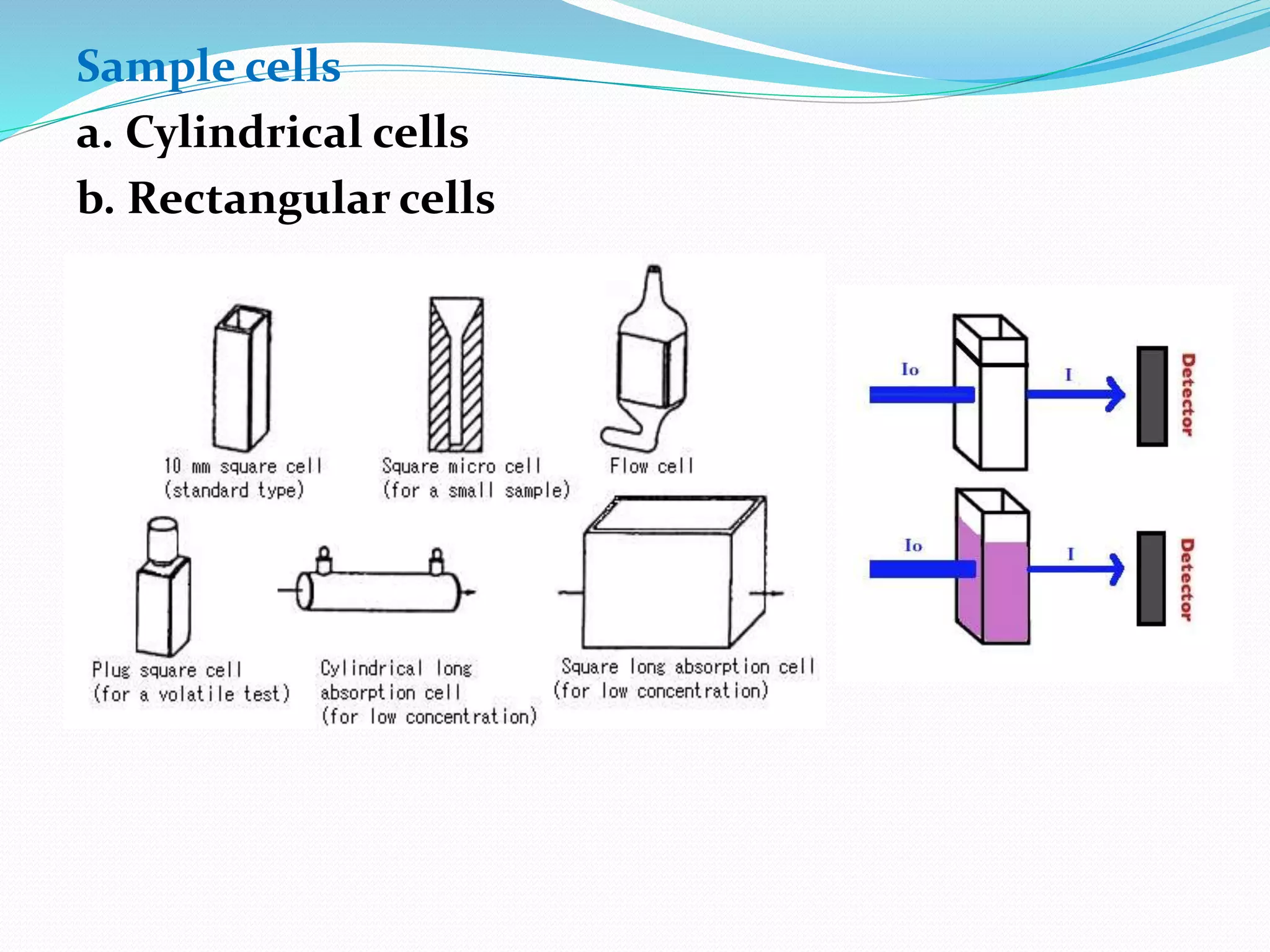
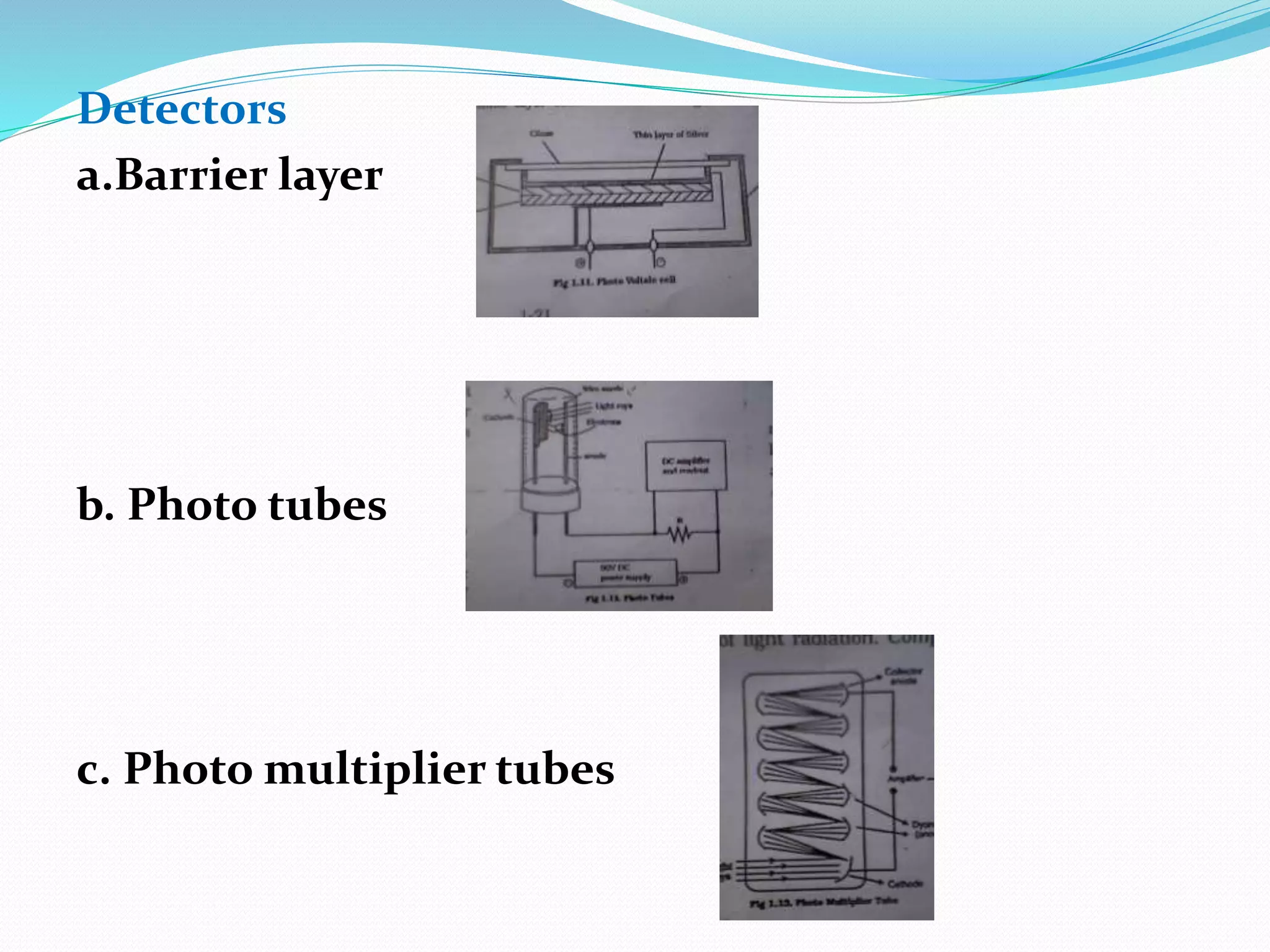
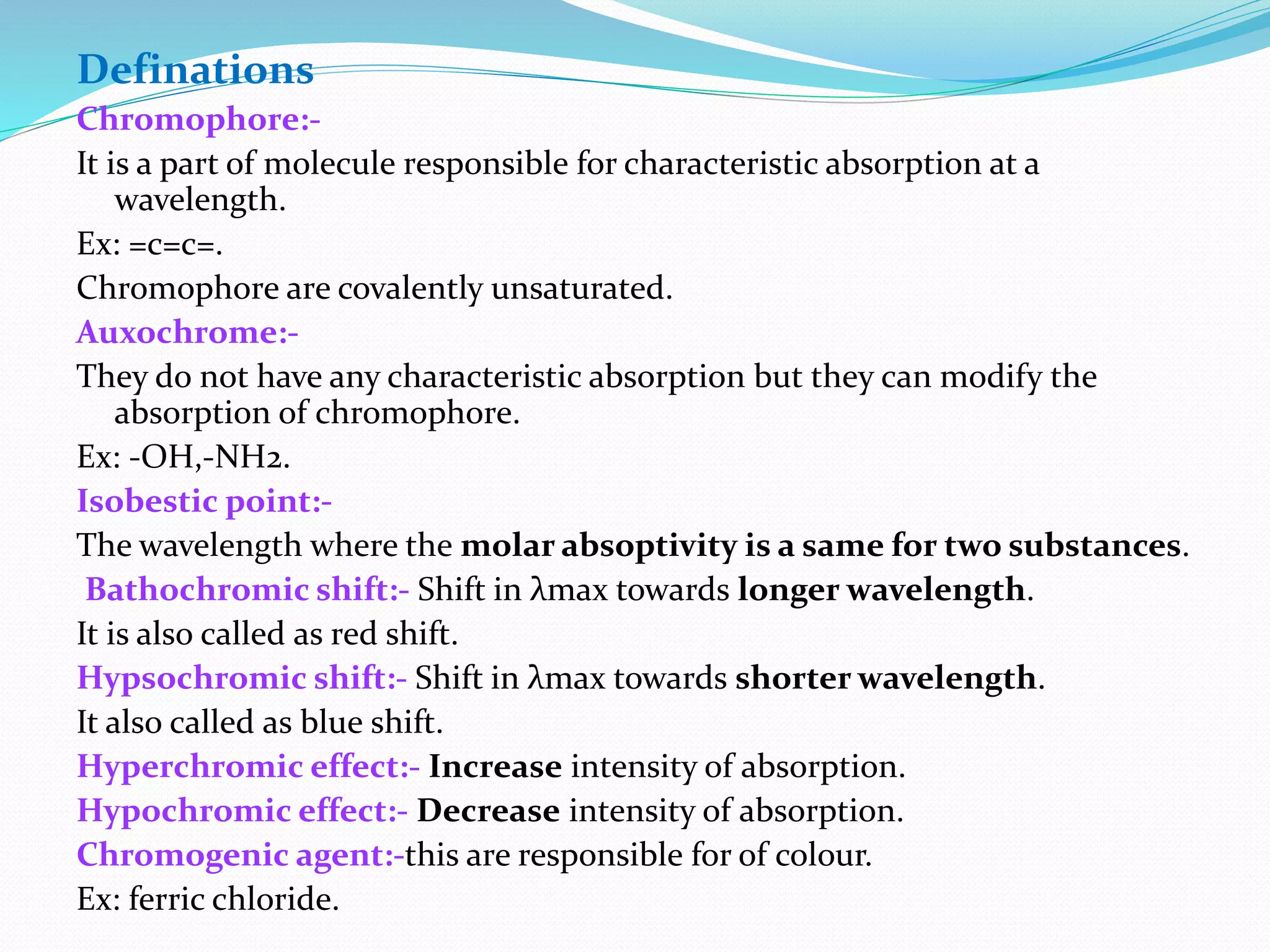
This document discusses visible spectroscopy, which analyzes absorption of visible light. It can determine drug concentrations by measuring absorbance at different wavelengths. Key aspects covered include Beer's and Lambert's absorption laws, common instrumentation like light sources, filters, monochromators, sample cells and detectors. Applications include purity testing, quantitative analysis, and determining functional groups or molecular weights. Key terms defined are chromophore, auxochrome, isobestic point, and bathochromic, hypsochromic, hyperchromic, and hypochromic effects.