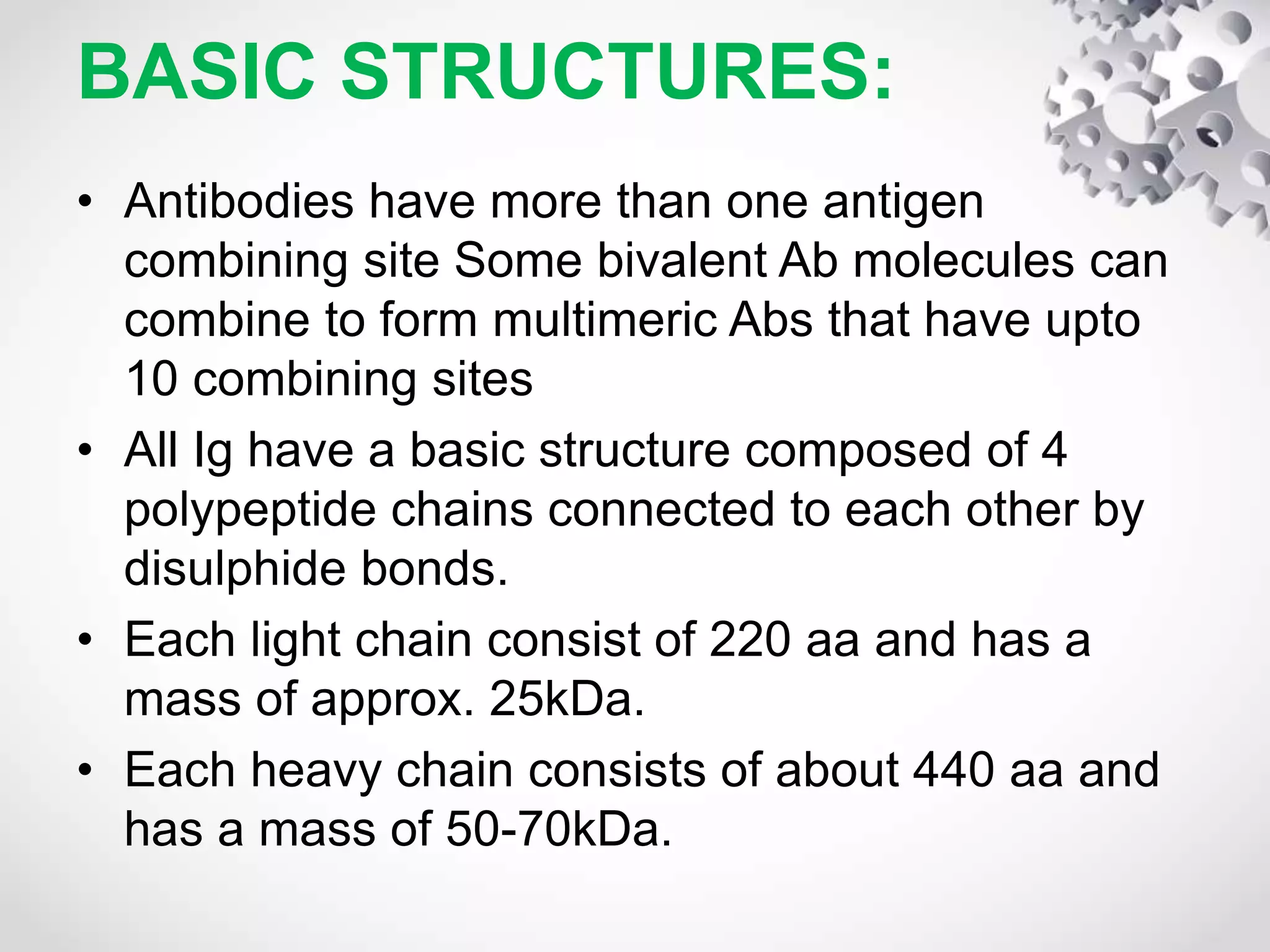
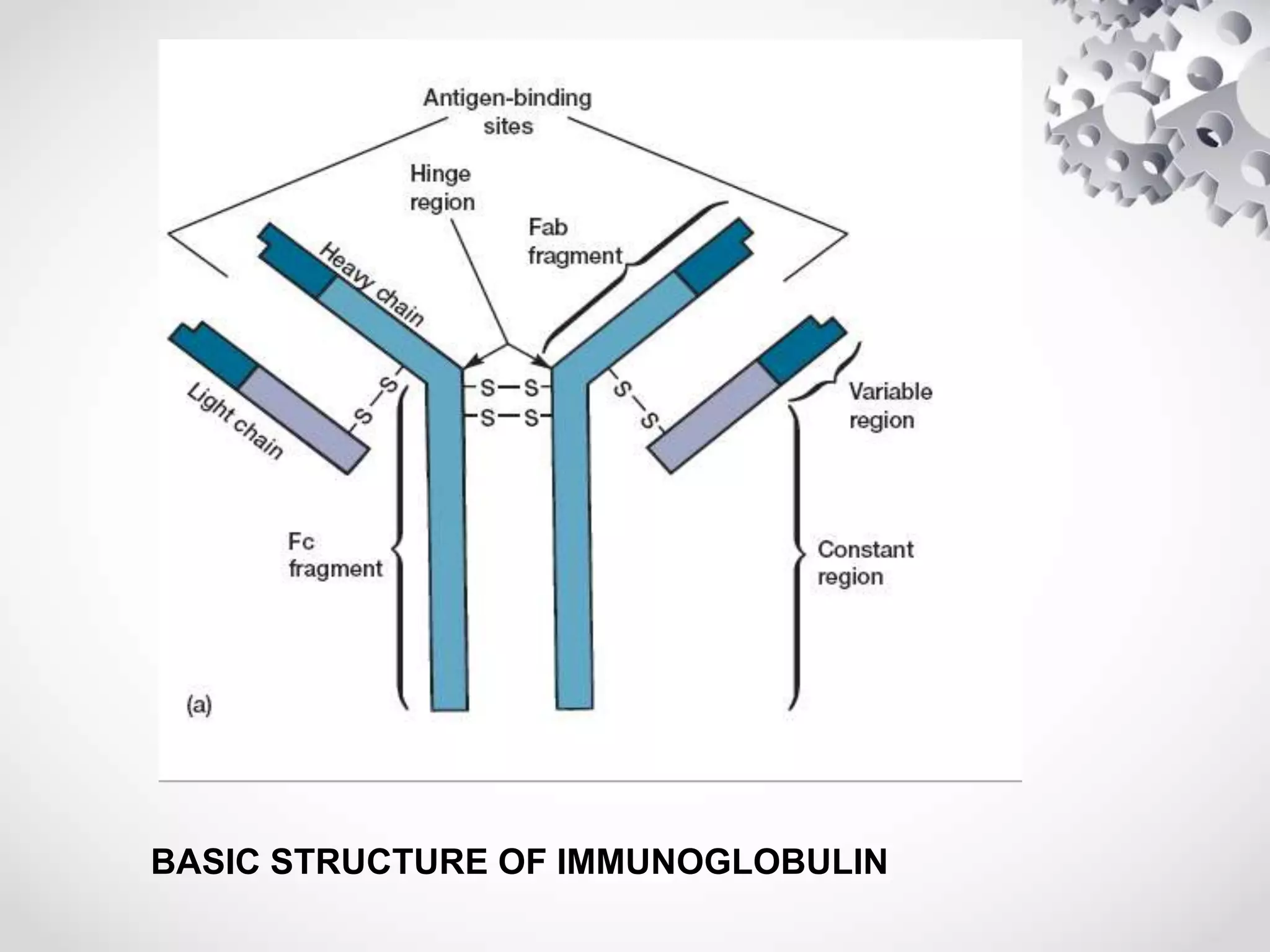
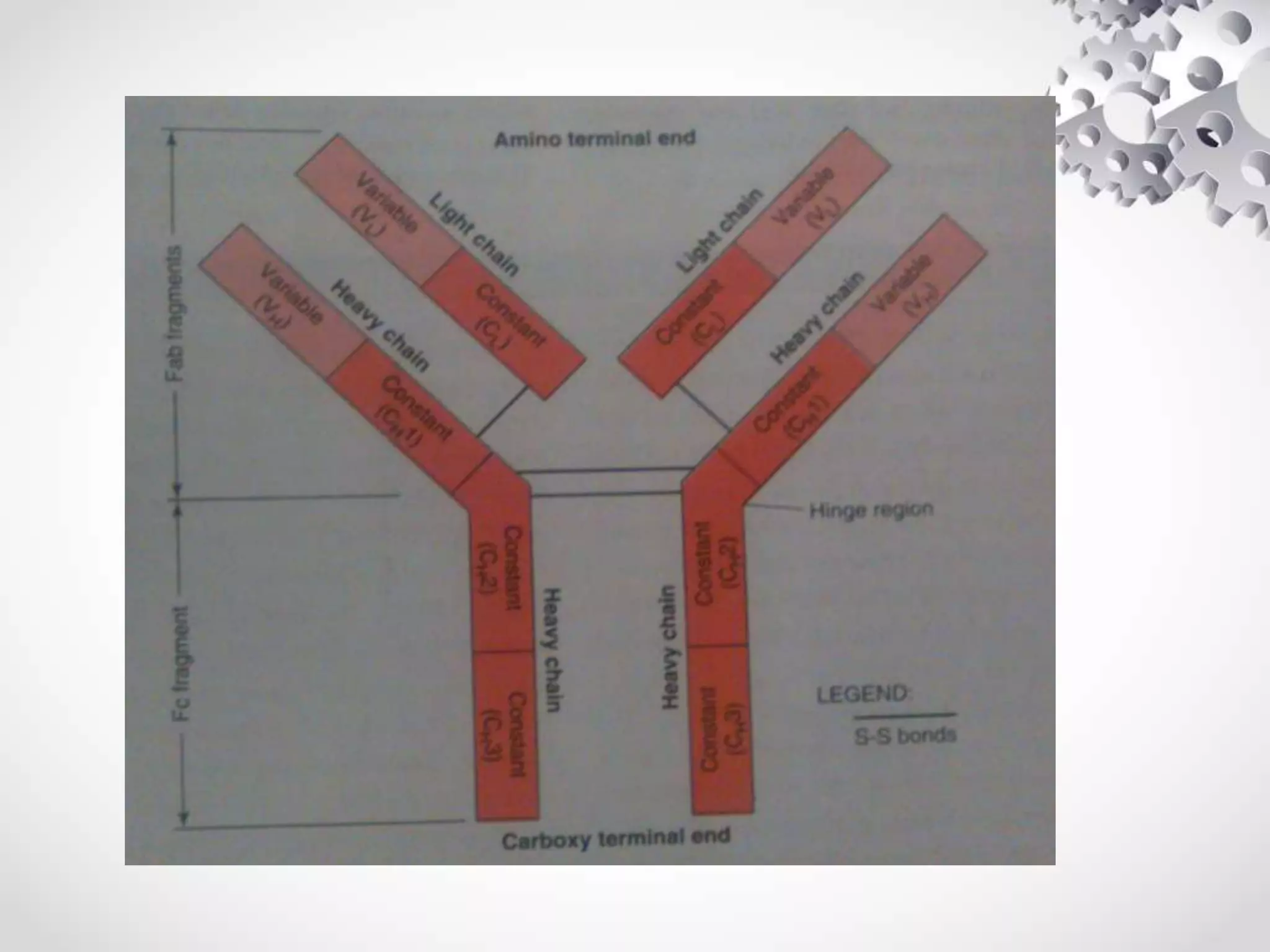
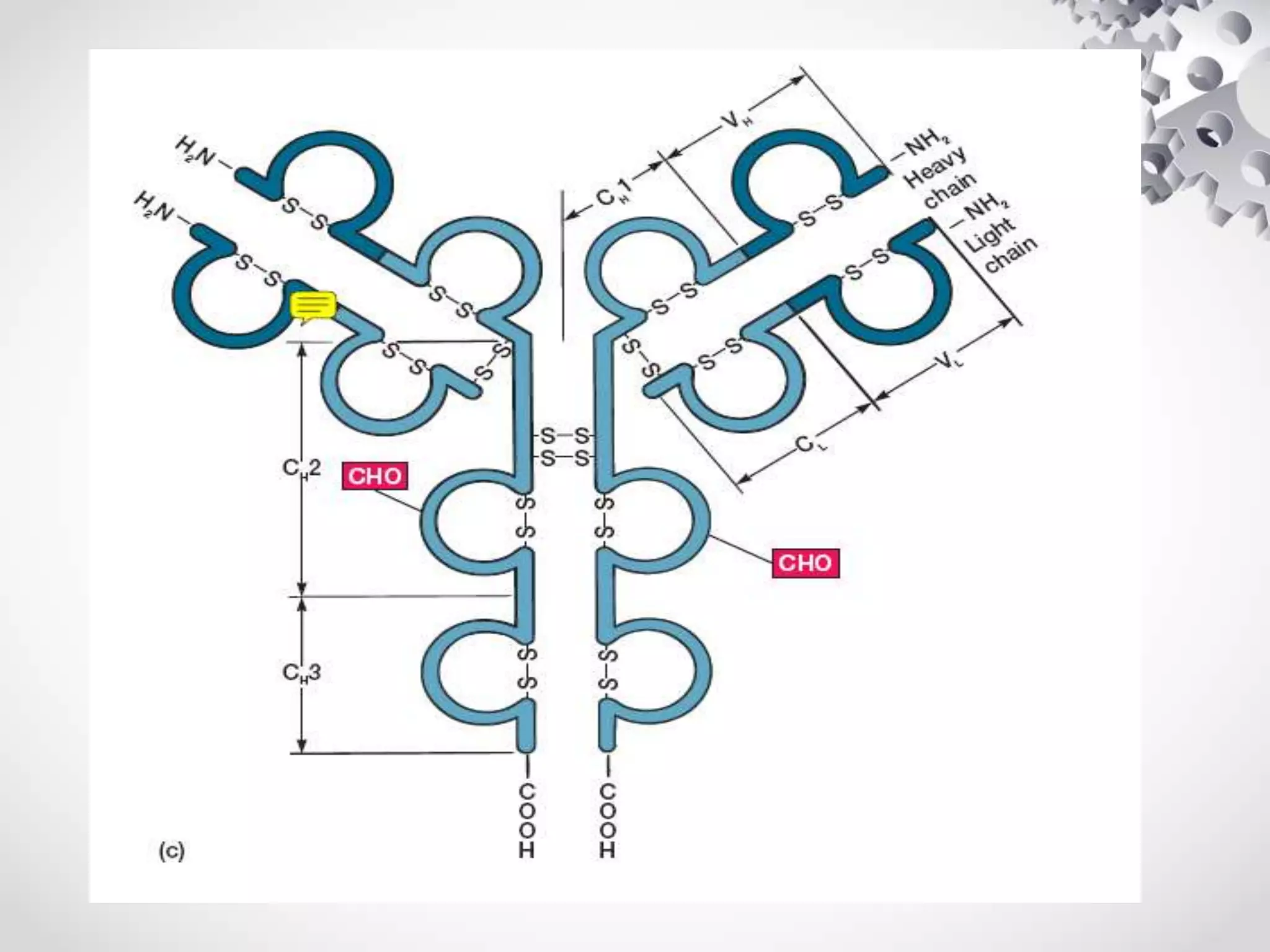
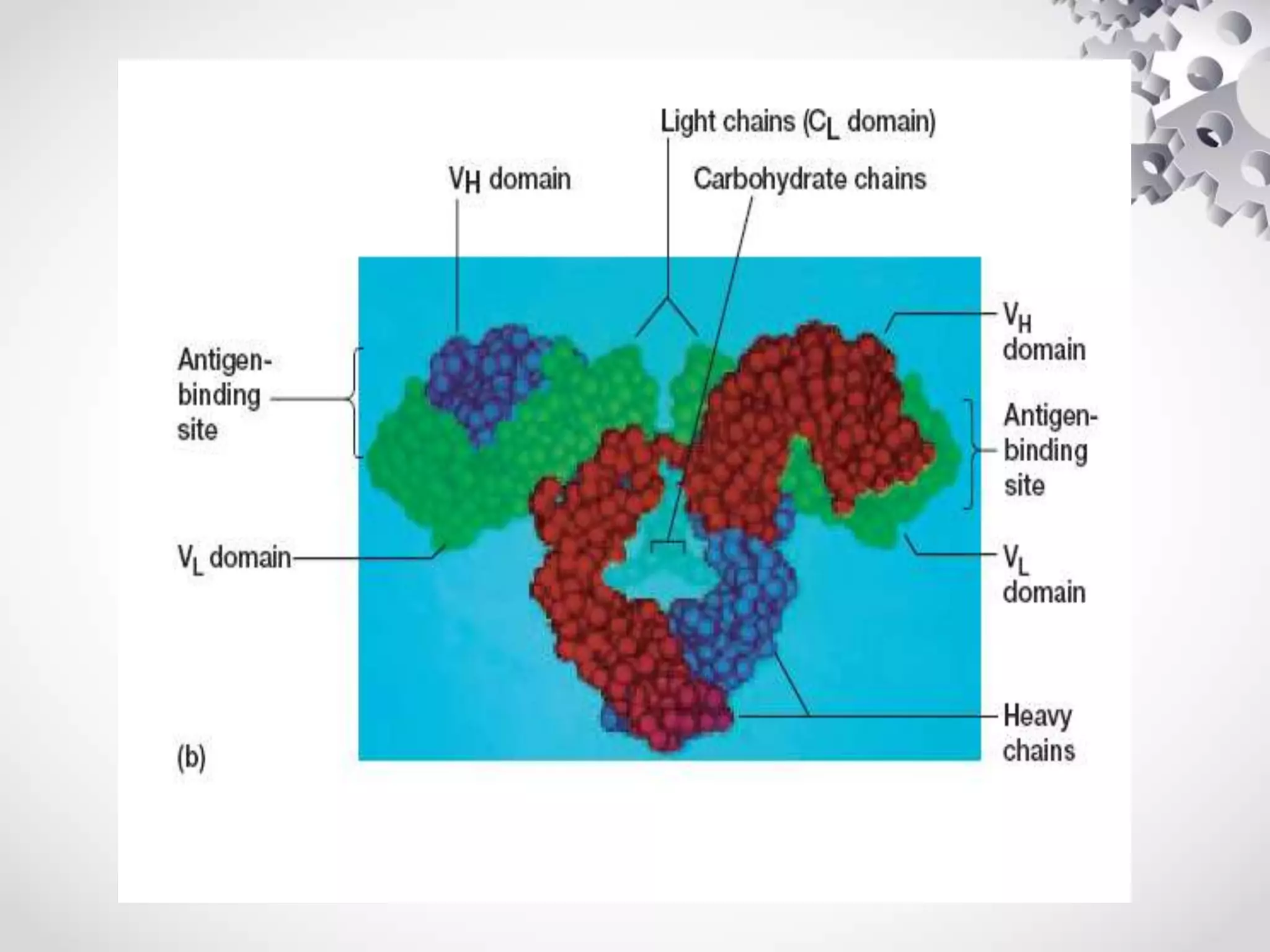
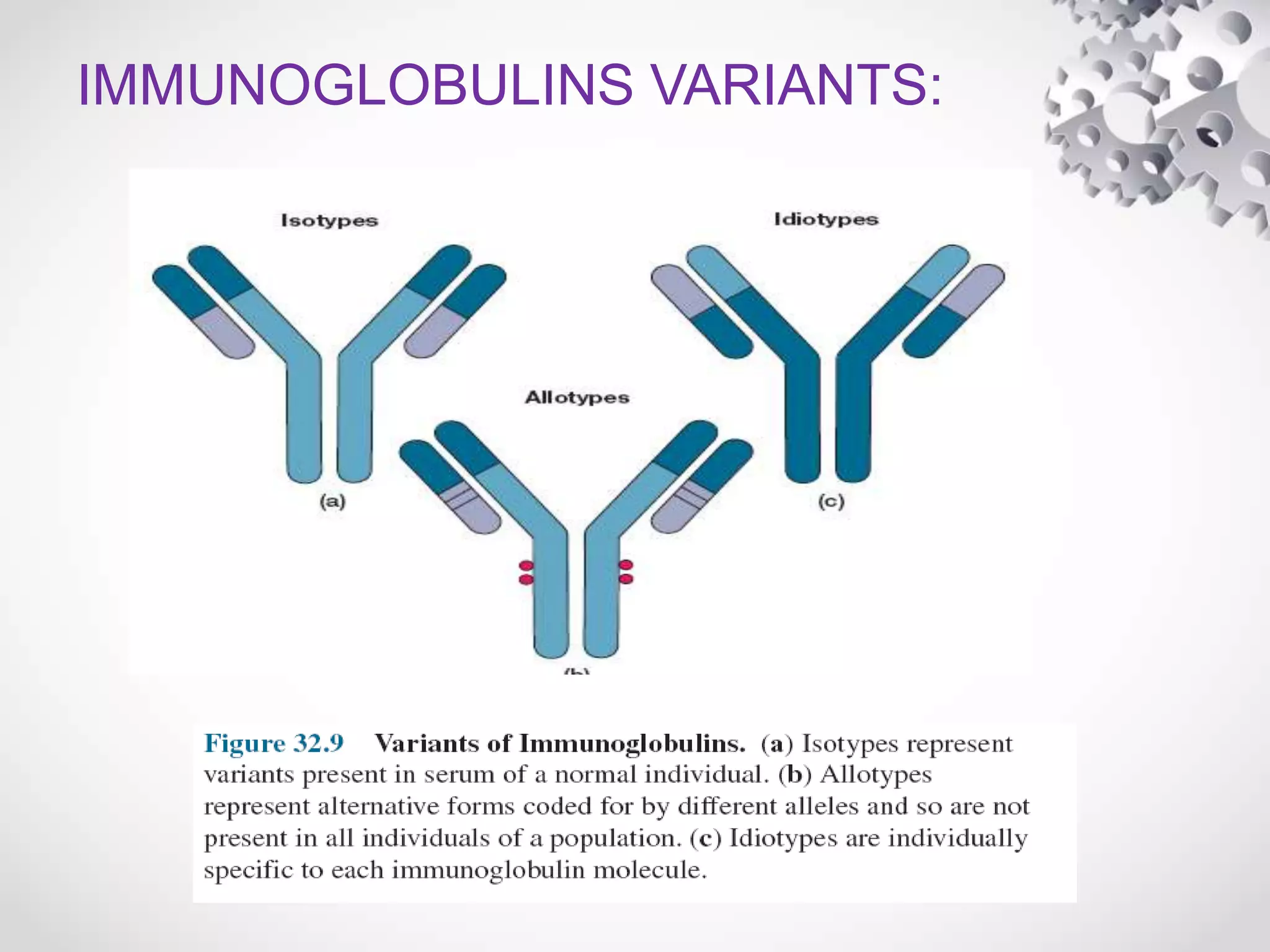
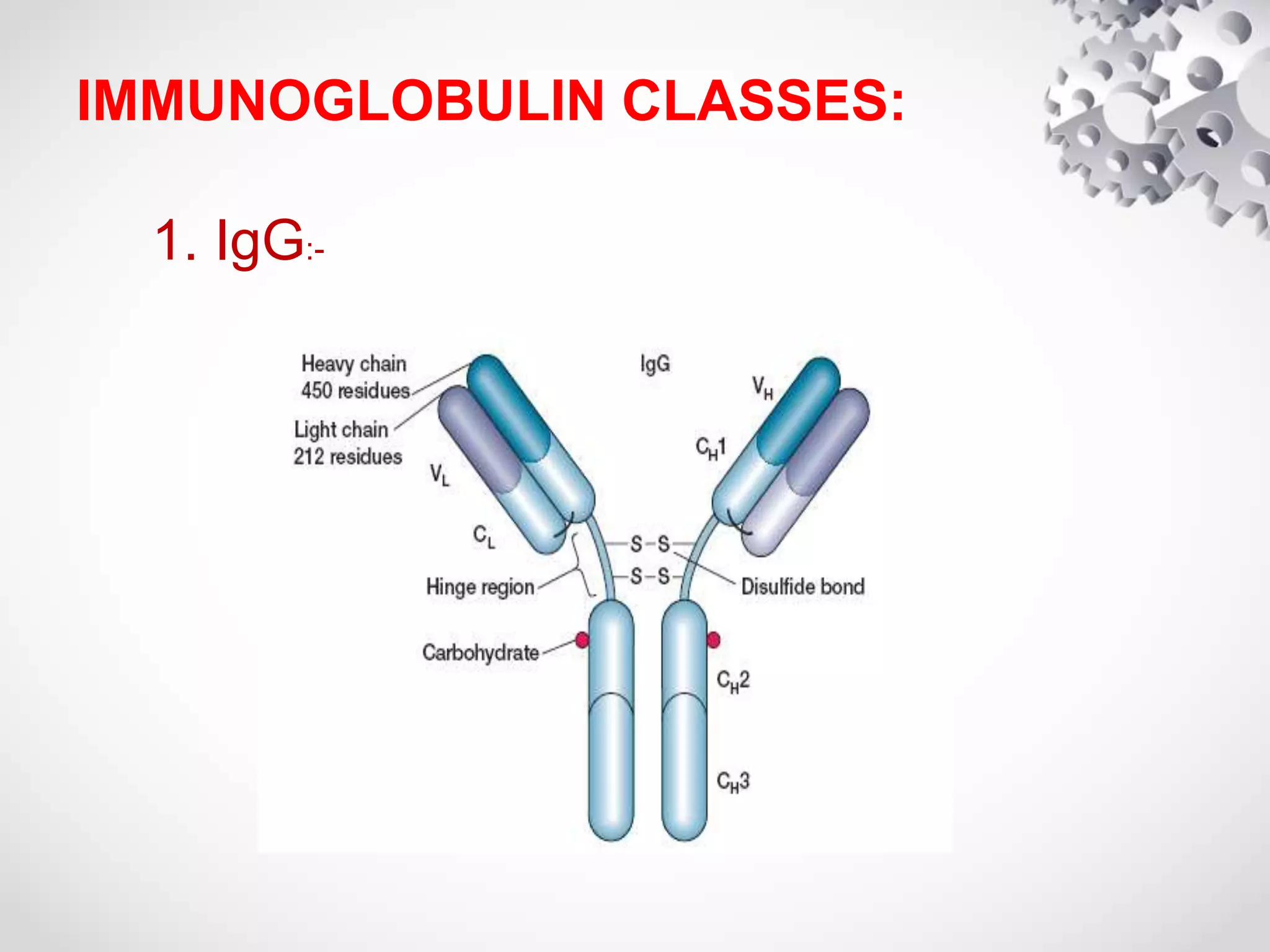
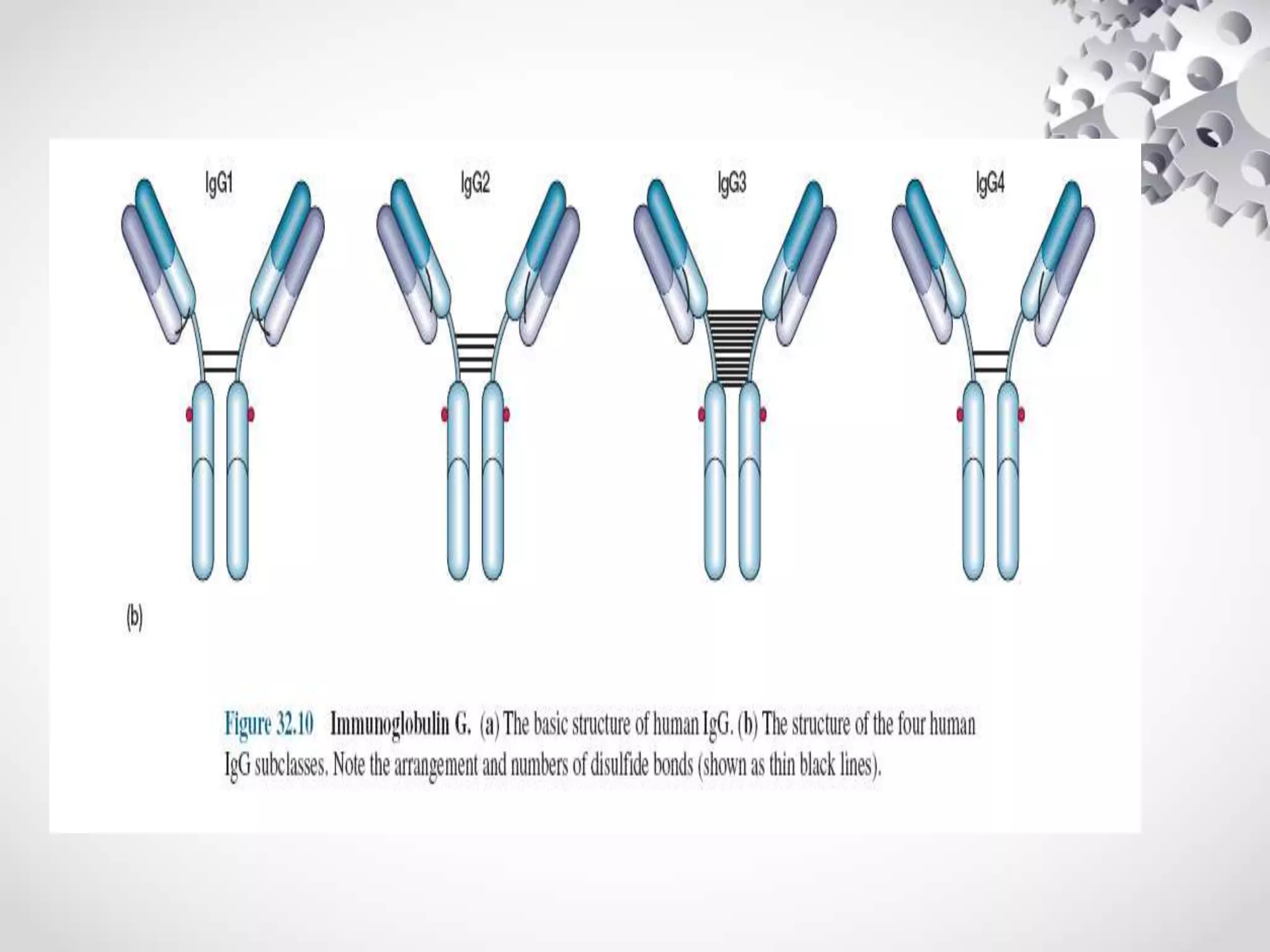
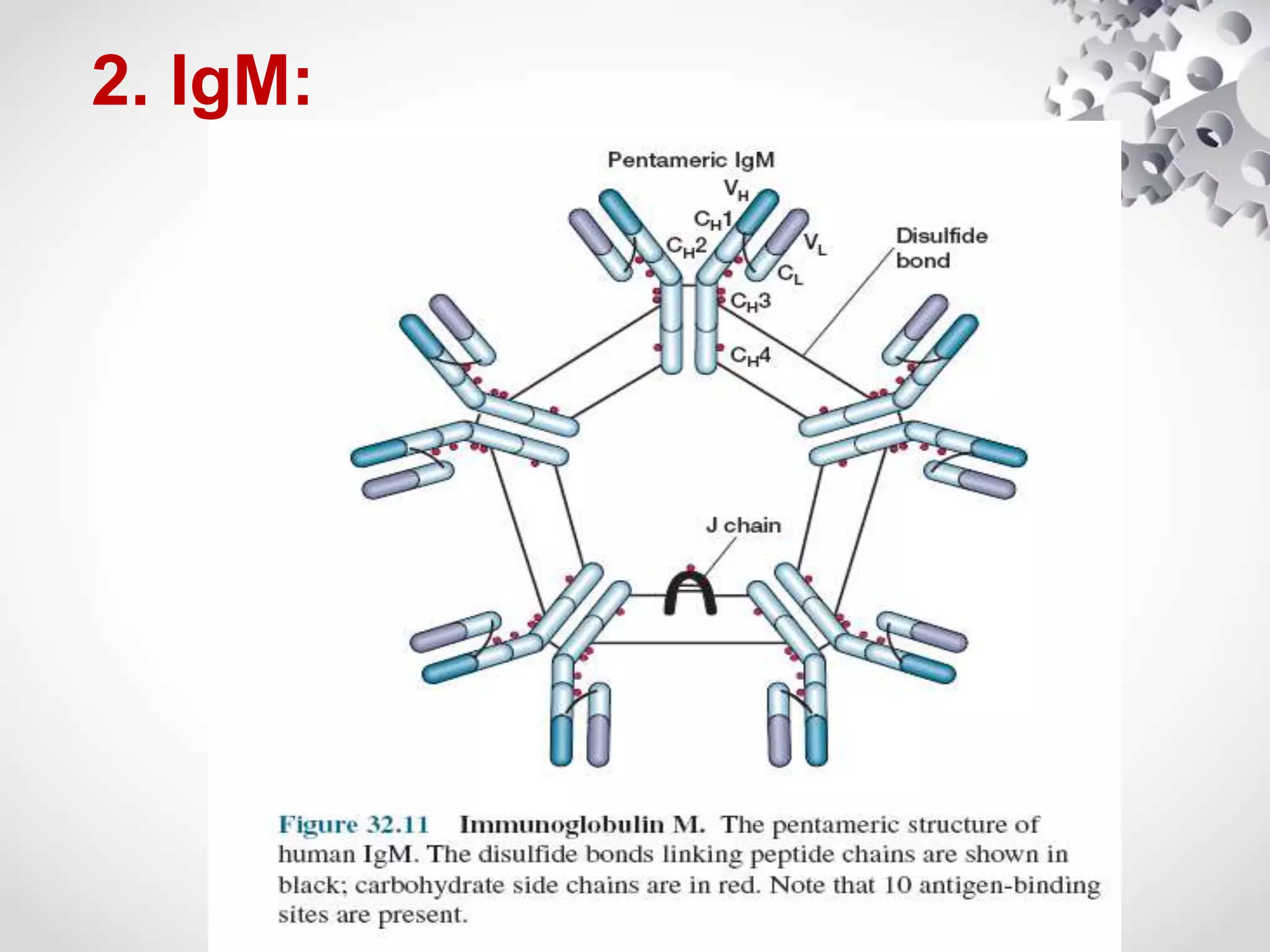
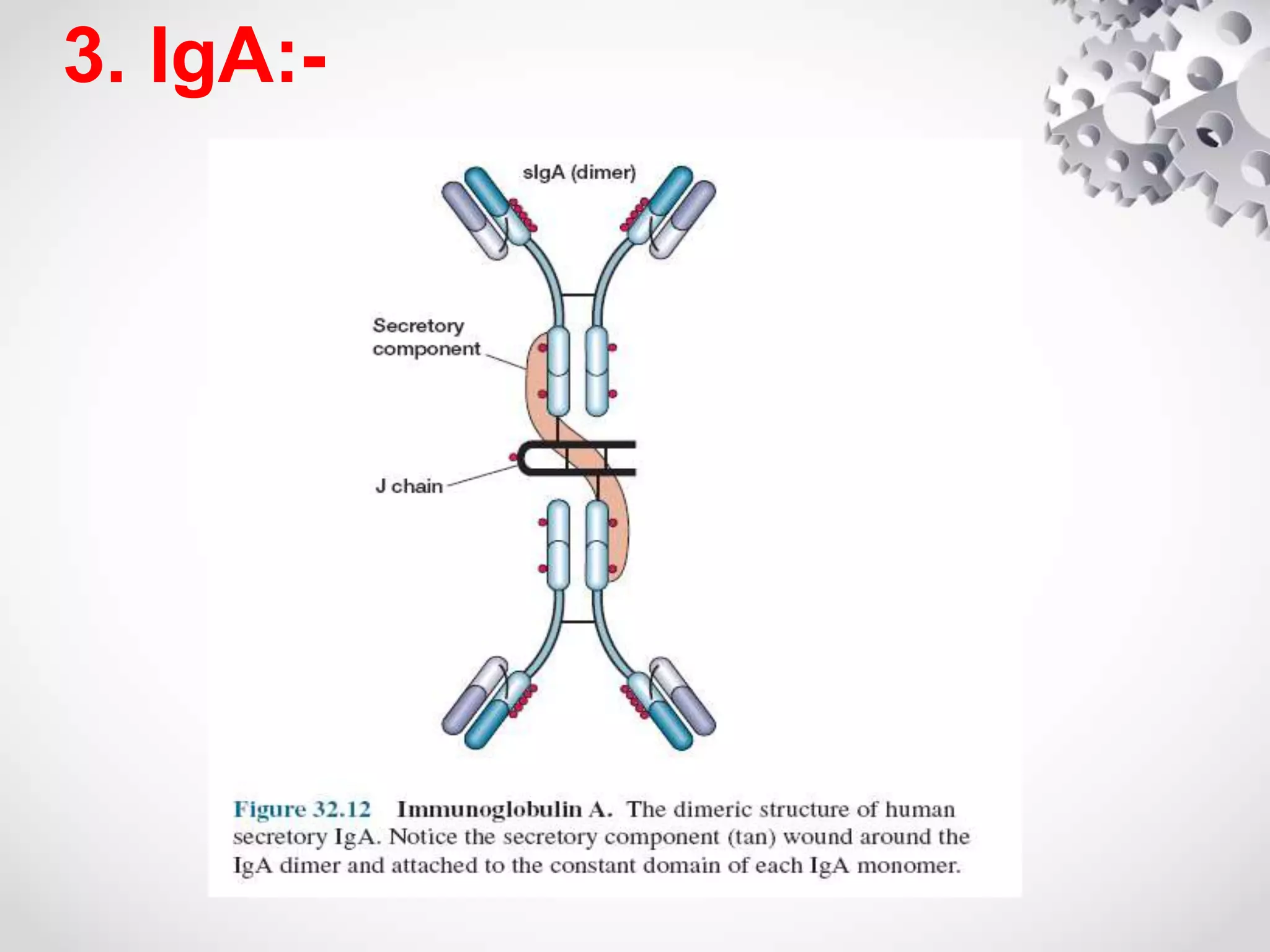
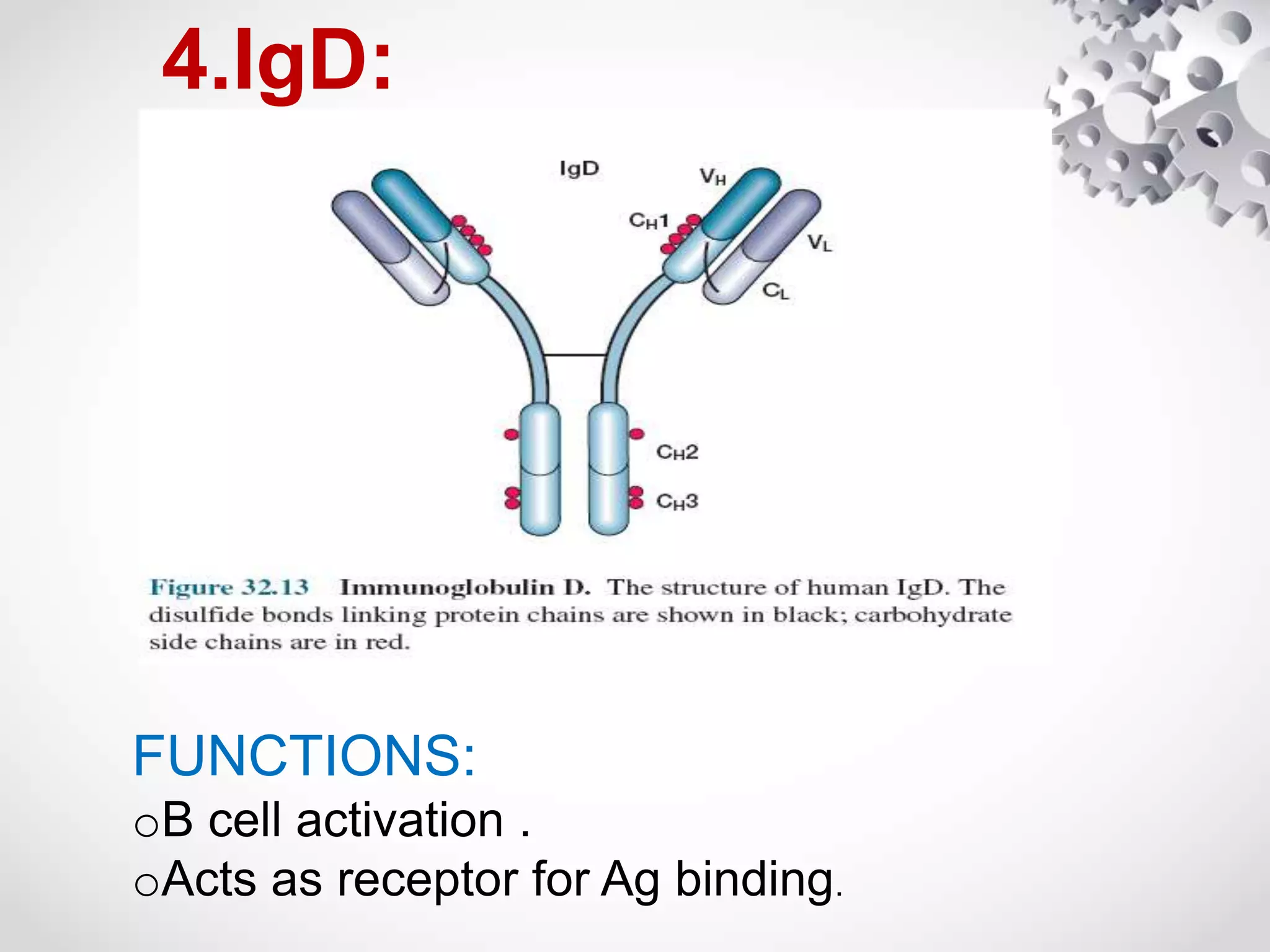
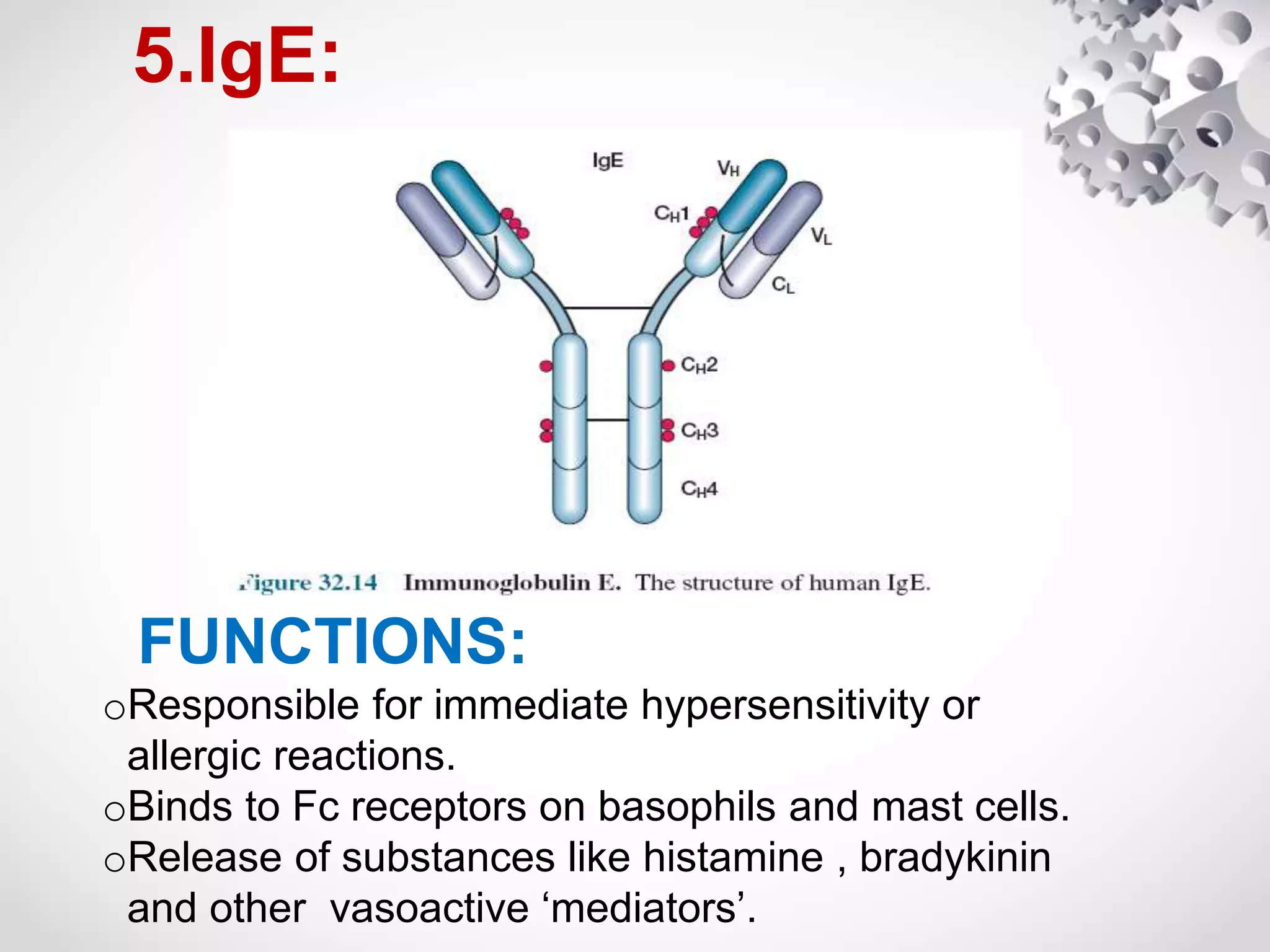
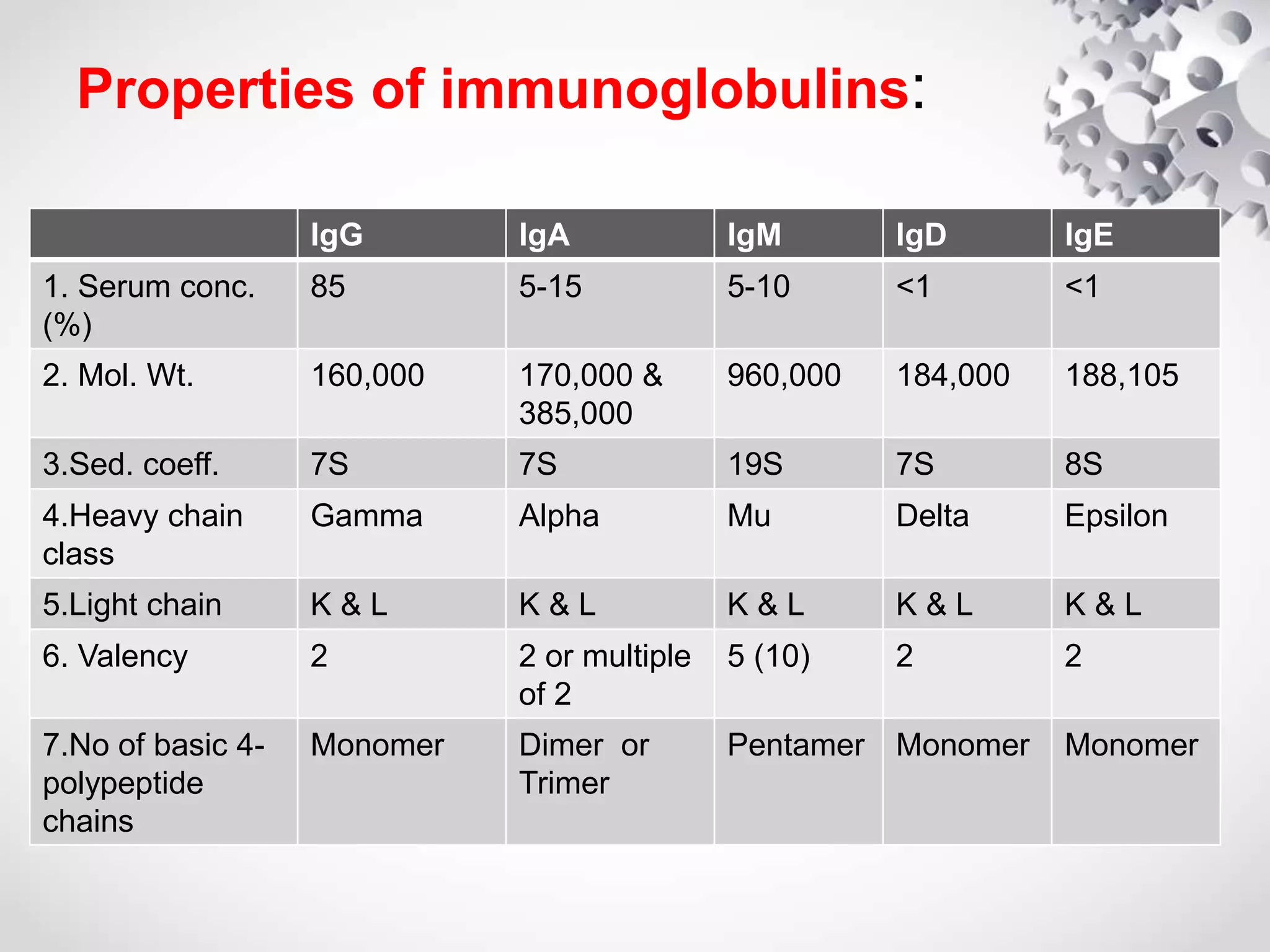
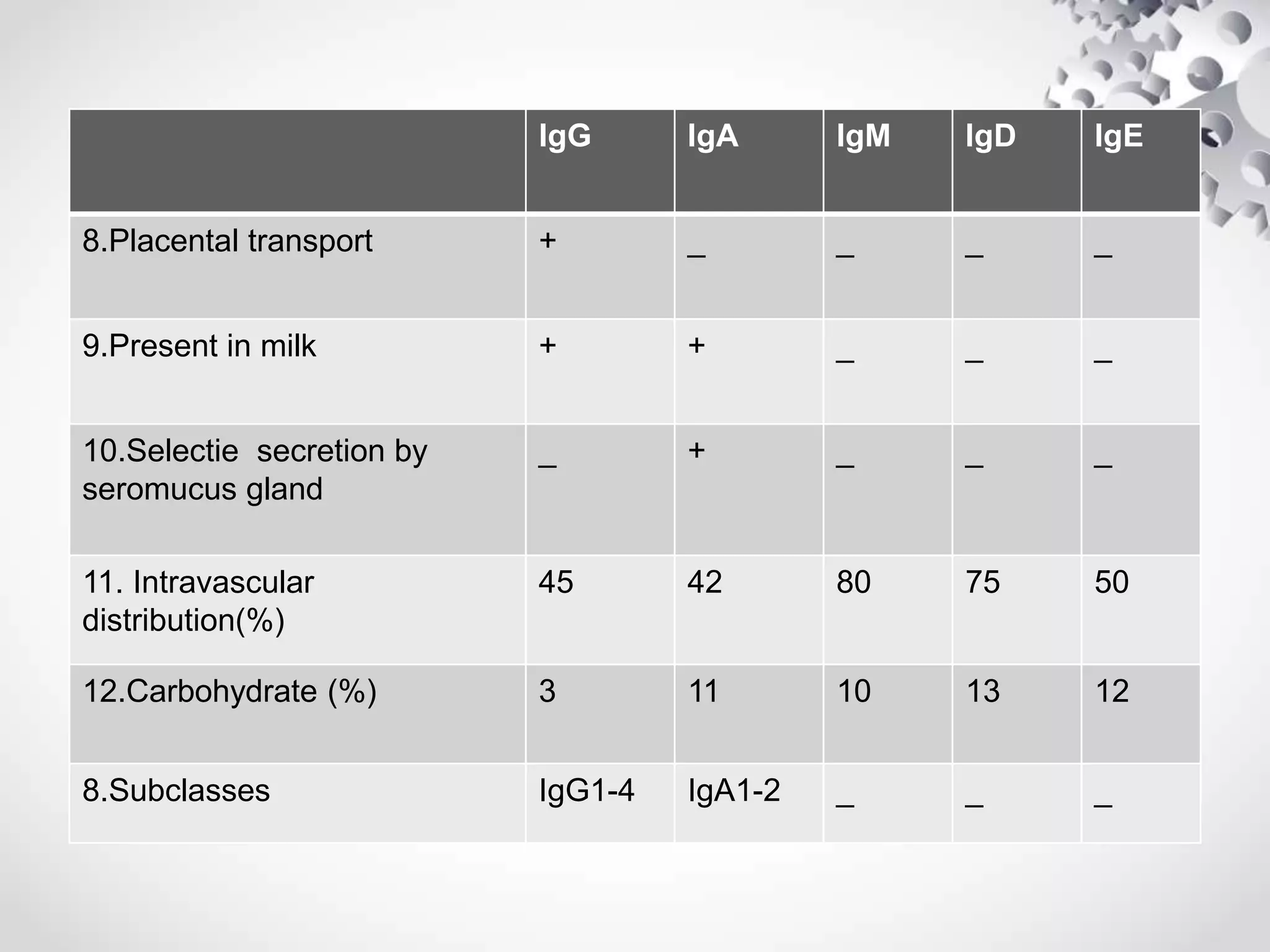
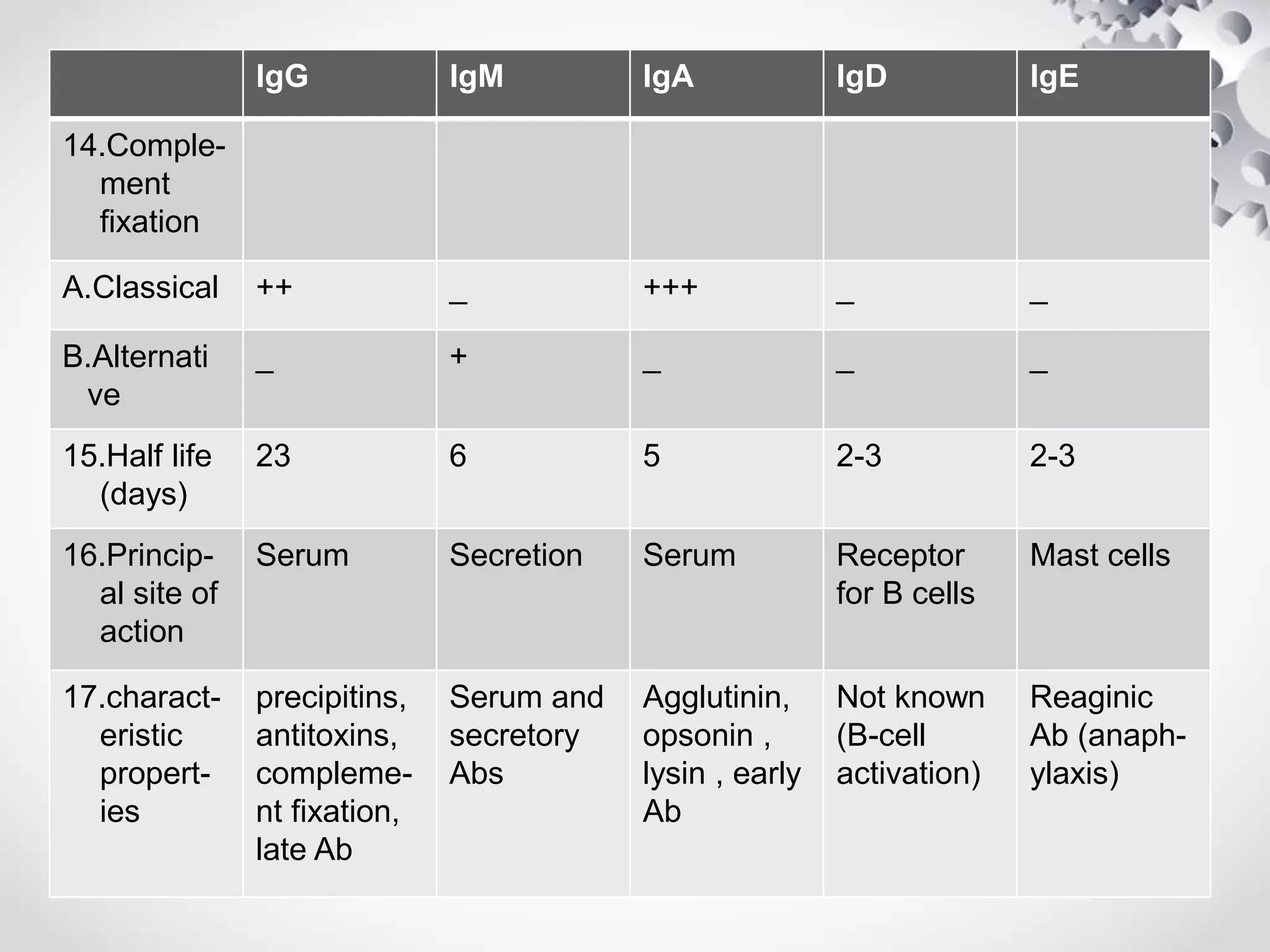
An antibody, also known as an immunoglobulin, is a glycoprotein produced by plasma cells in response to an antigen. Antibodies have a basic Y-shaped structure composed of two light polypeptide chains and two heavy chains connected by disulfide bonds. The variable regions at the top of the Y allow antibodies to bind to specific antigens, while the constant region mediates other immune functions. The five major classes of antibodies are IgG, IgA, IgM, IgD, and IgE, which have different structures, functions, and roles in the immune response. IgG is the most abundant antibody and provides long-term immunity, while IgM acts as the first line of defense and IgA protects mucos