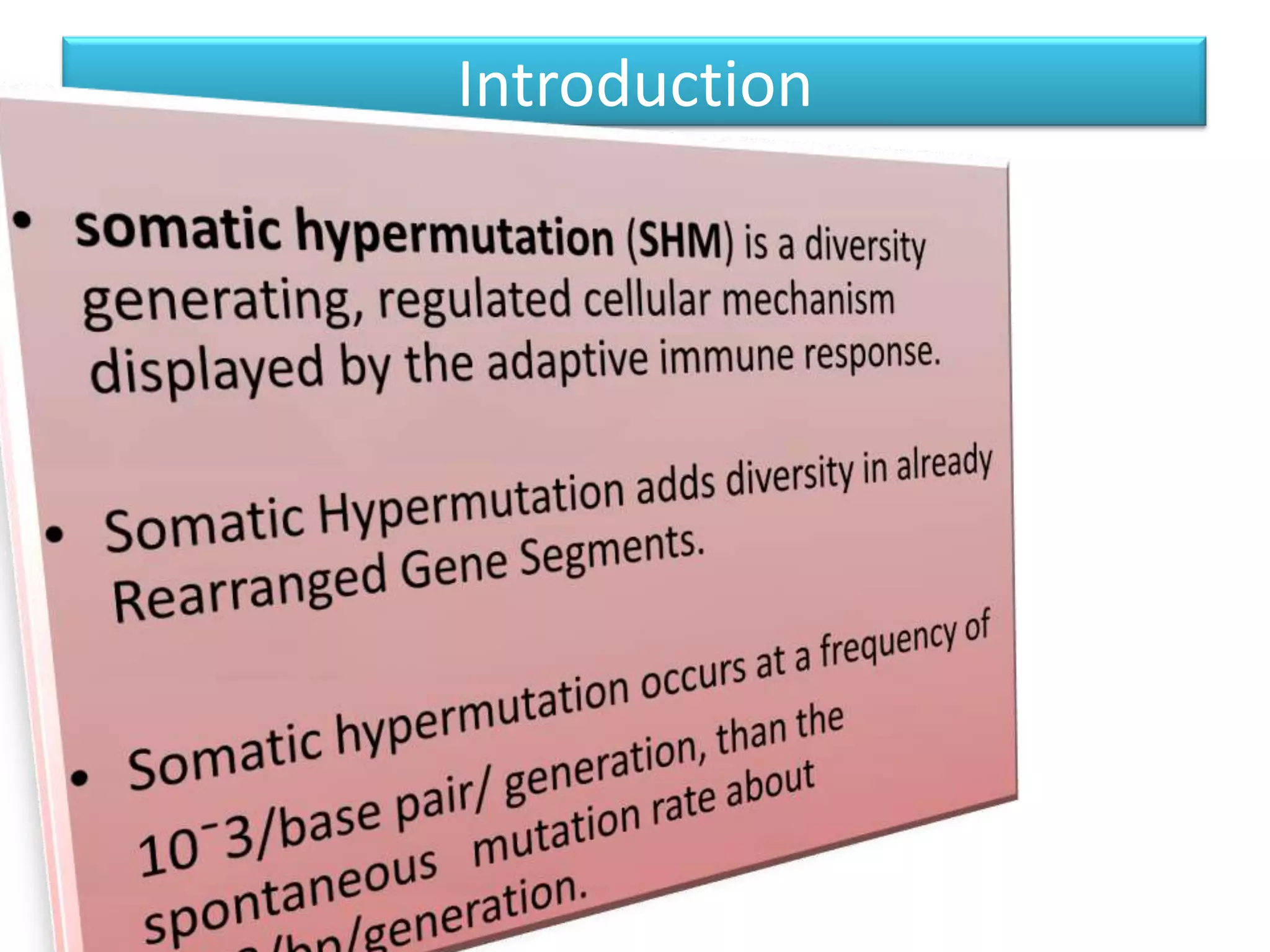
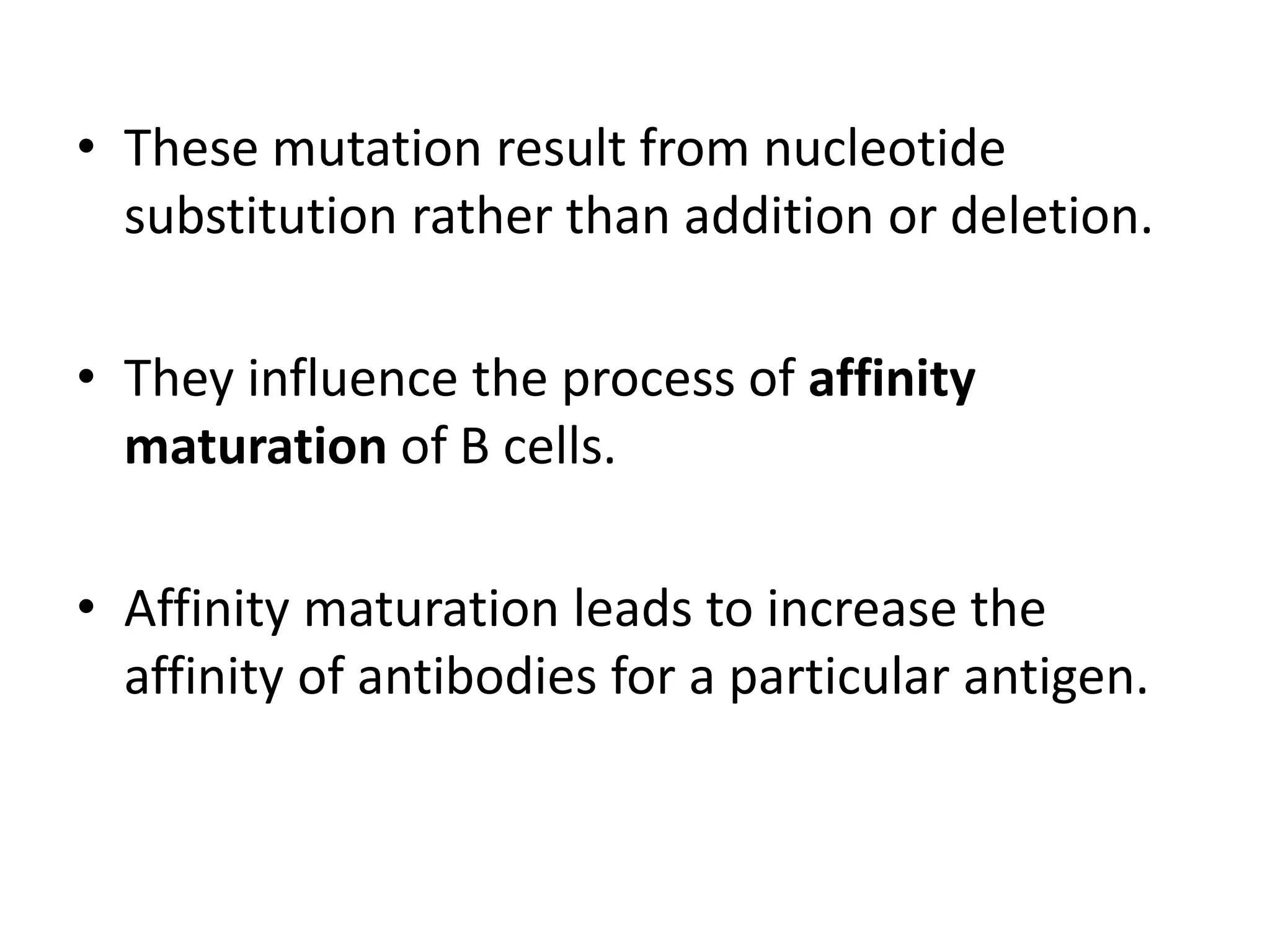
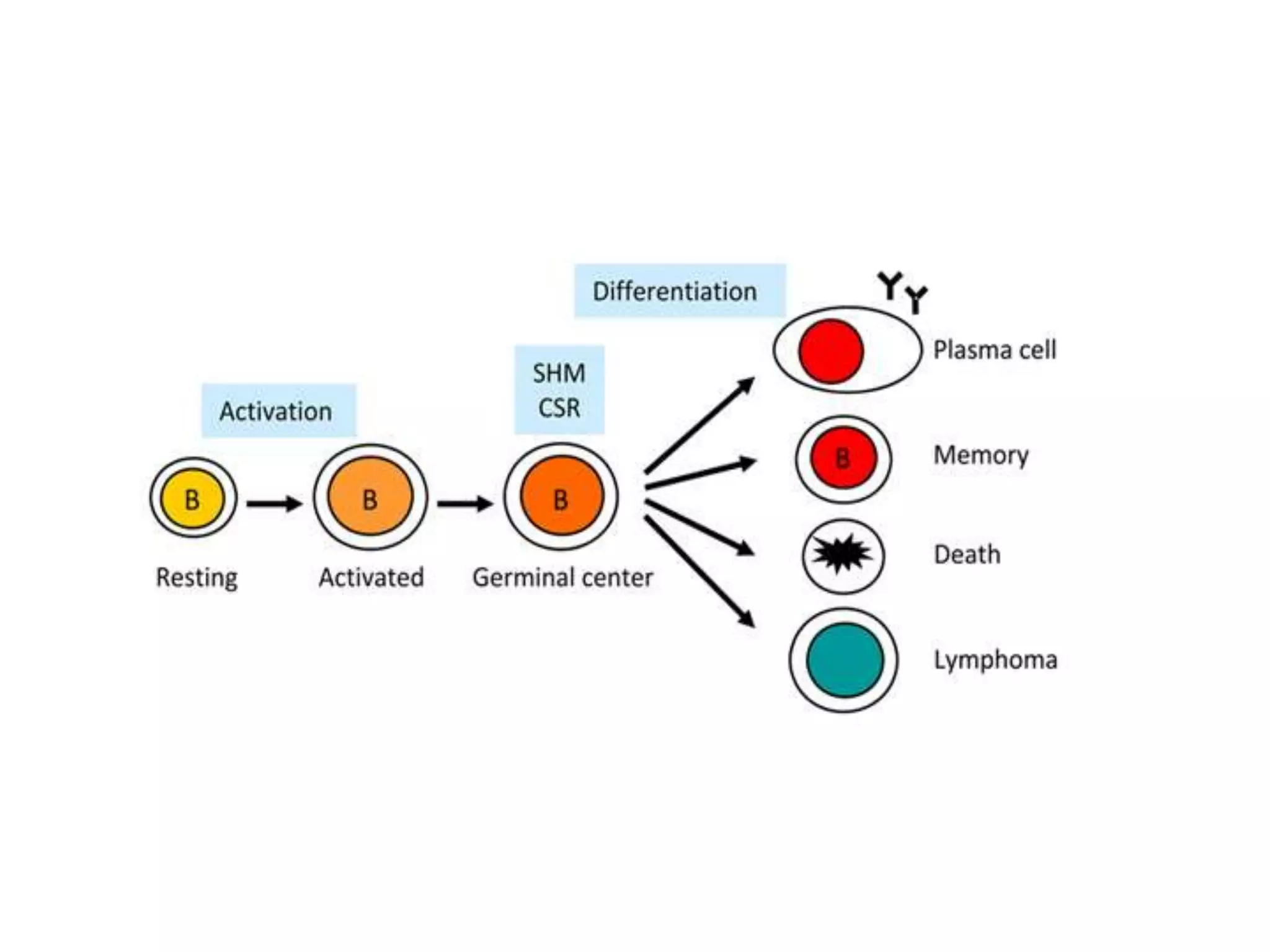
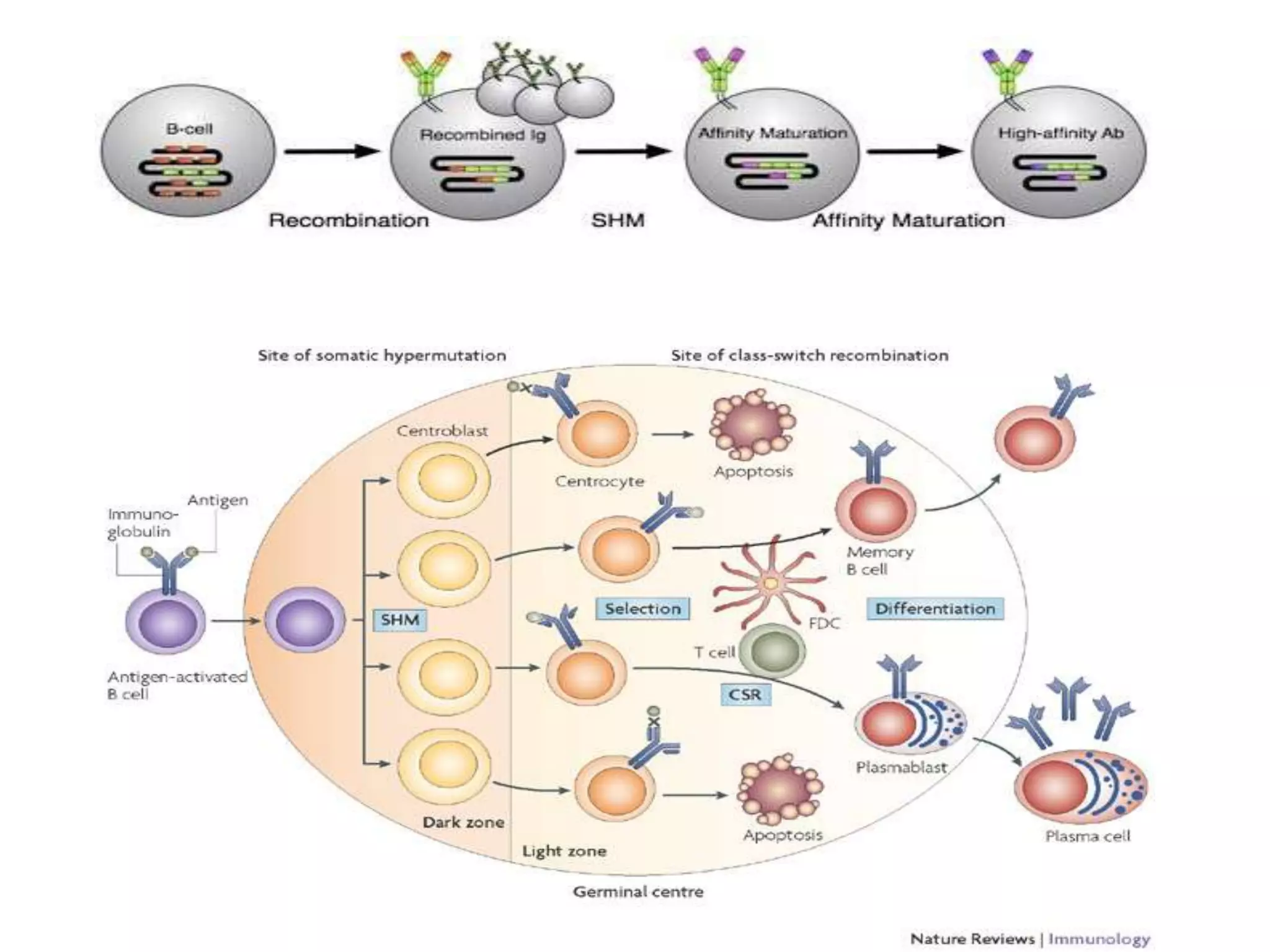
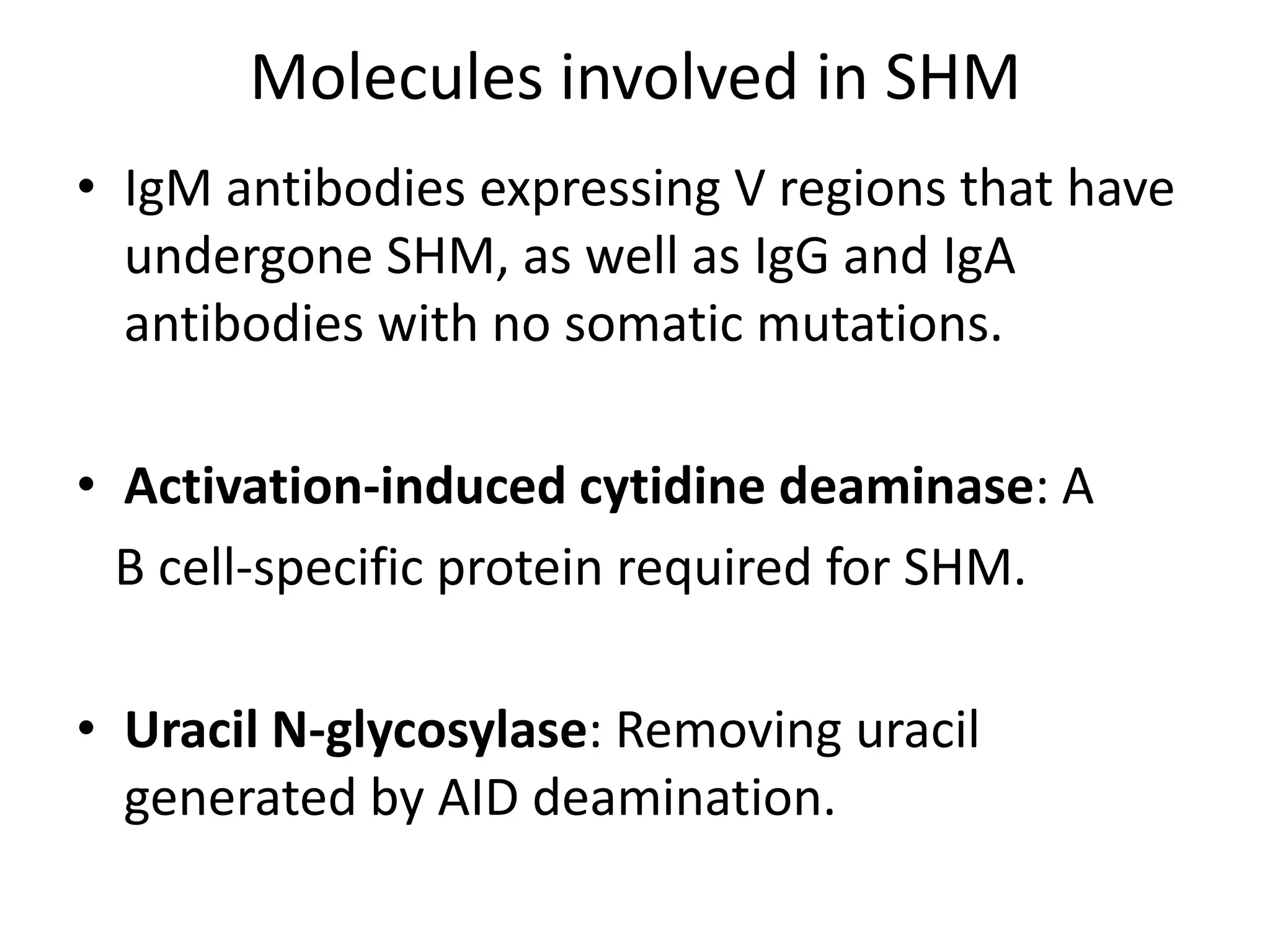
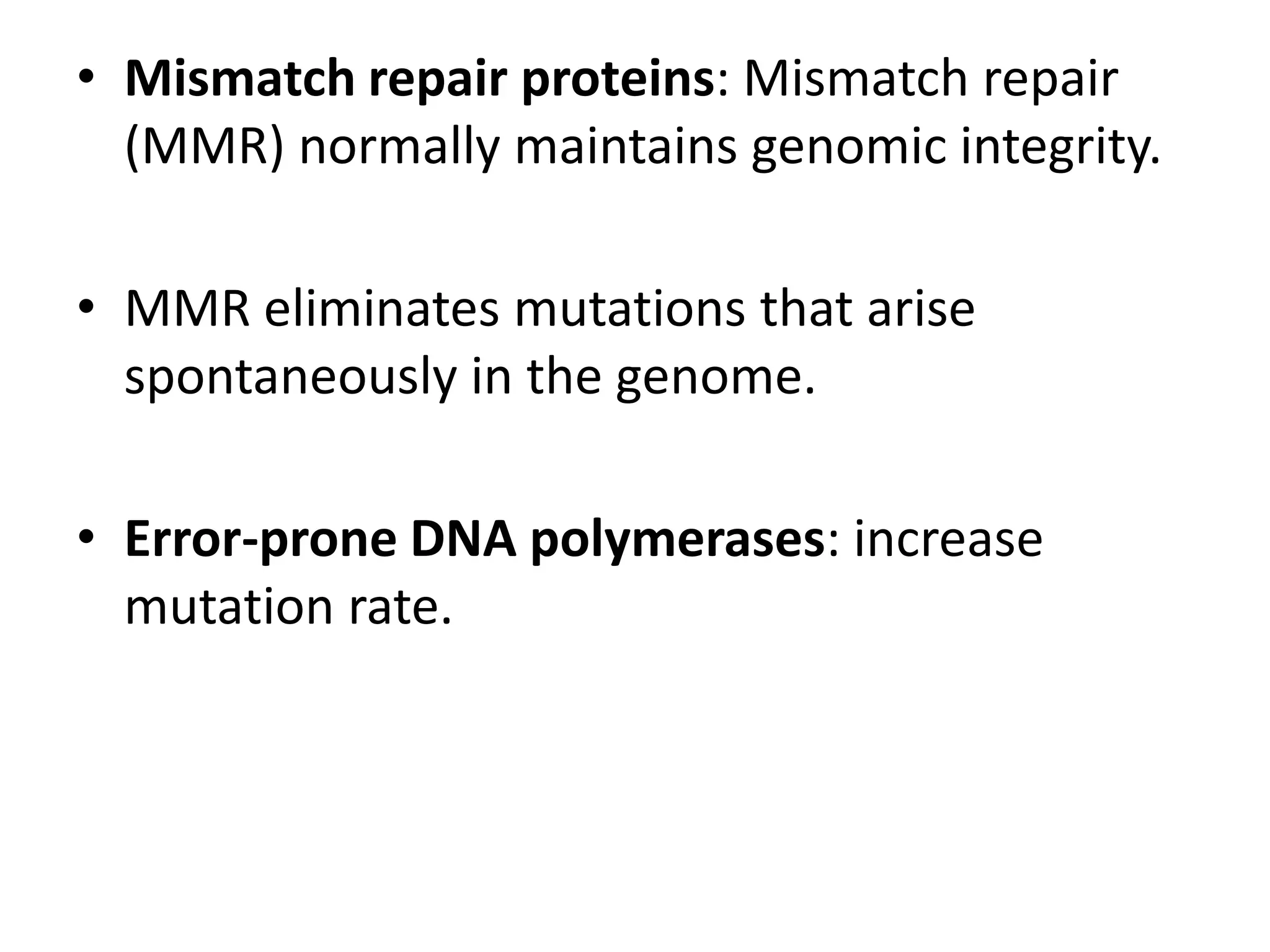
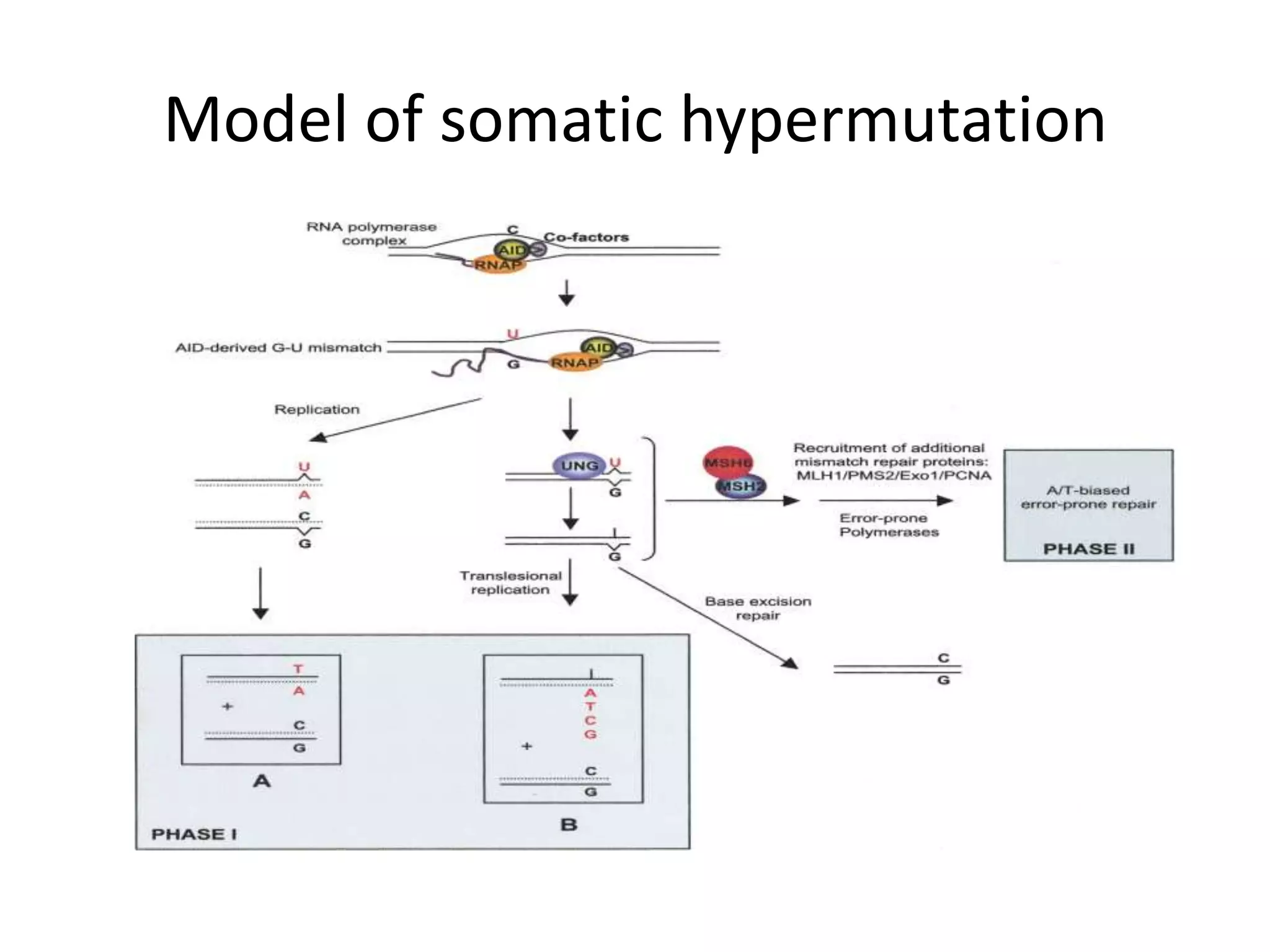
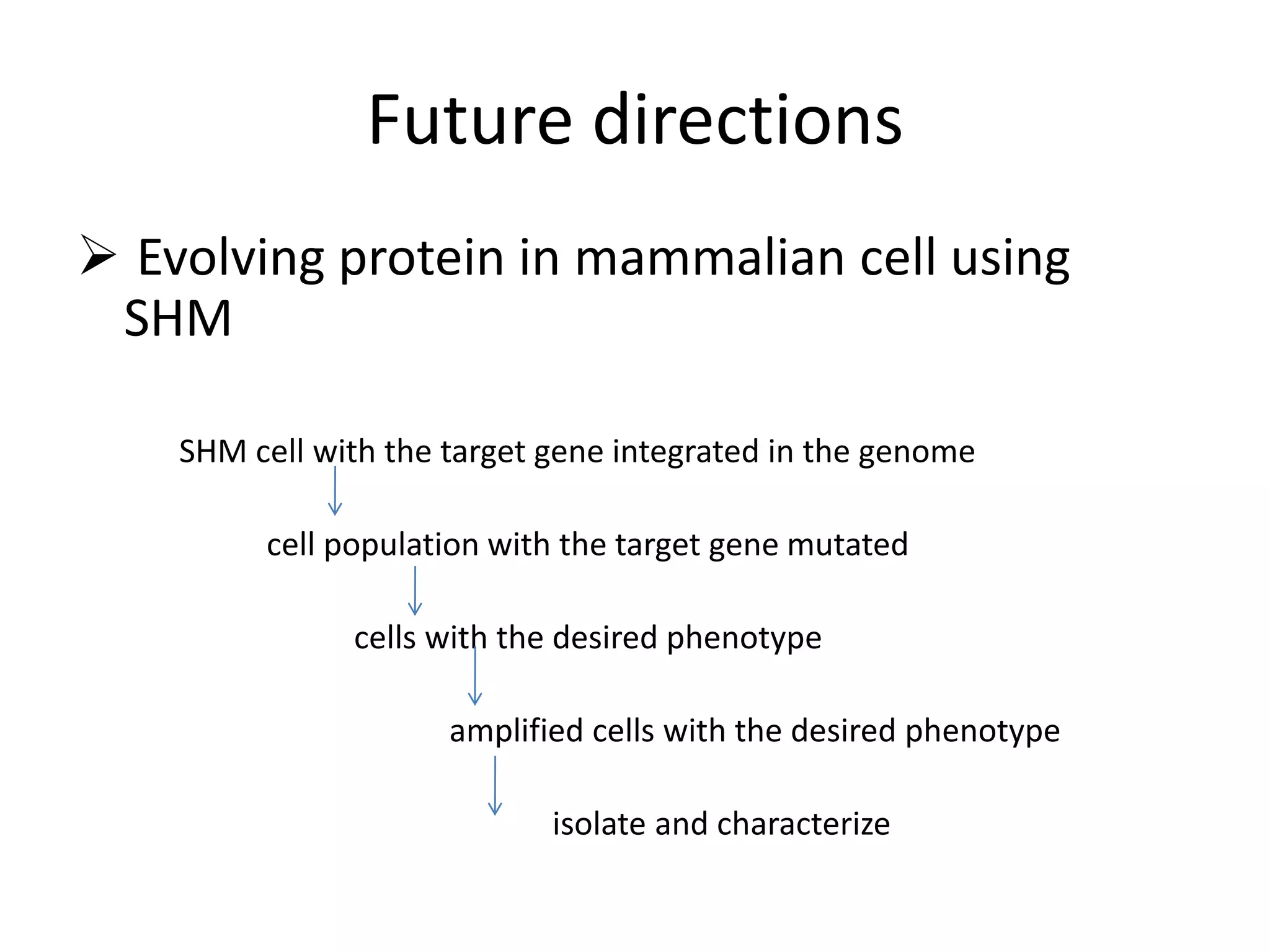
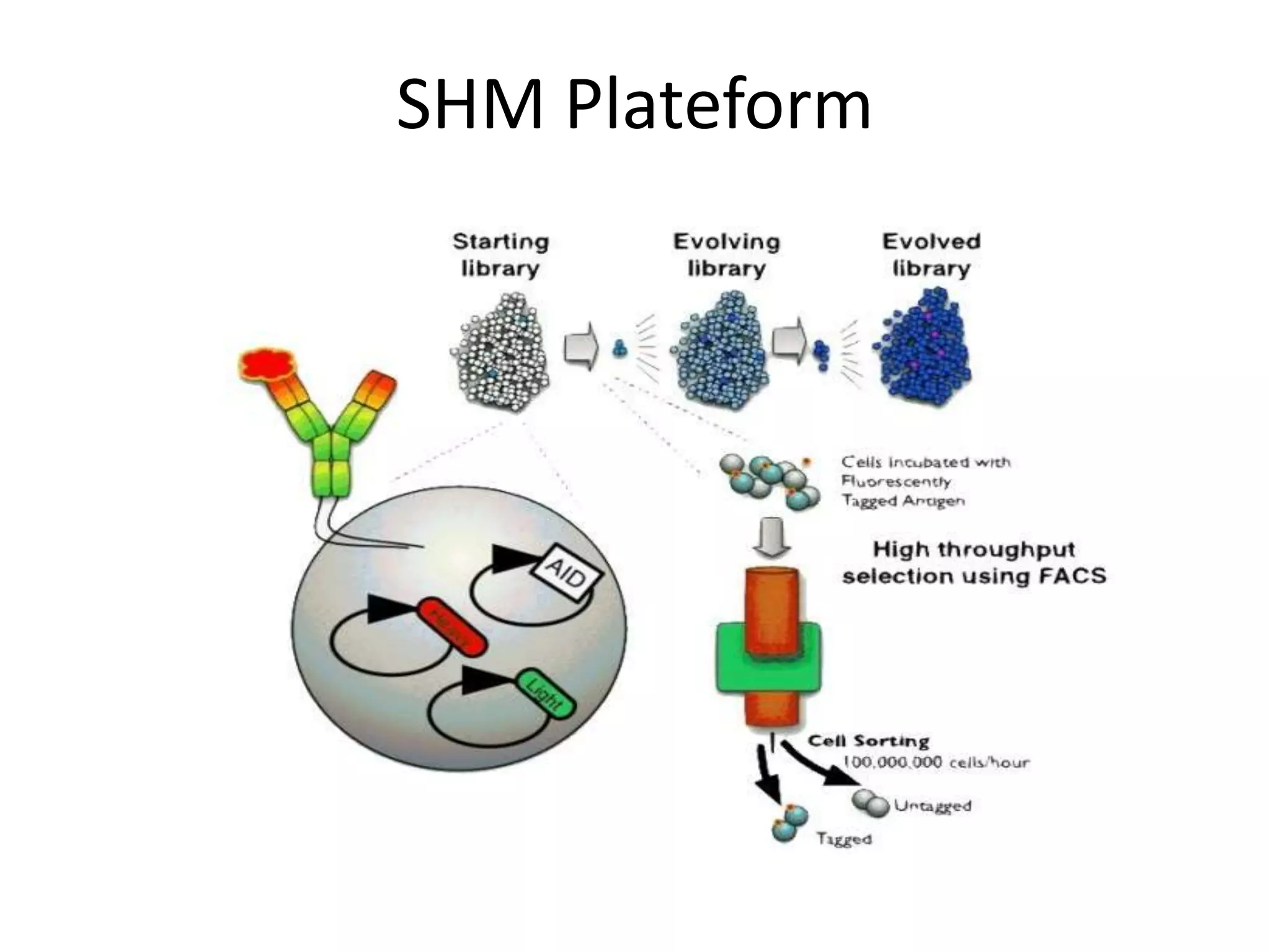
Somatic hypermutation (SHM) introduces mutations in antibody genes in B cells, increasing antibody diversity and affinity for antigens. SHM occurs through the actions of activation-induced cytidine deaminase and other molecules at a rate of 10-3 mutations per base pair, compared to the typical spontaneous mutation rate of 10-8 per base pair. These mutations mainly occur in hypervariable regions of antibody genes and result from nucleotide substitutions. SHM leads to affinity maturation of B cells and increased antibody affinity for antigens. Future directions include using the SHM process to evolve proteins in mammalian cells.