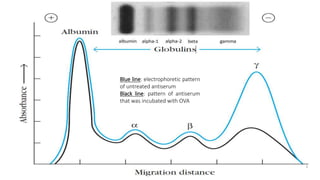
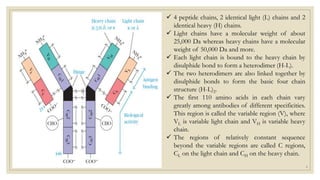
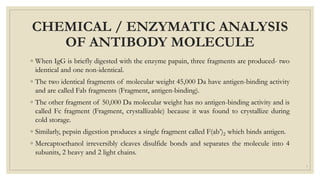
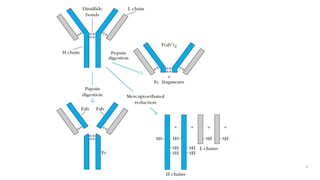
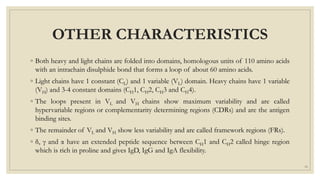
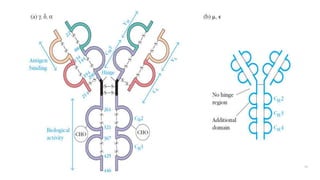
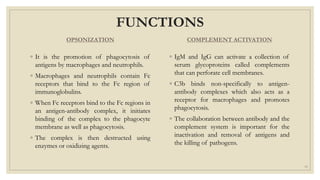
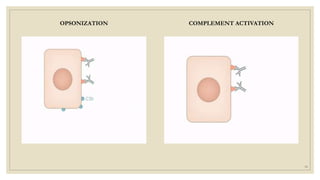
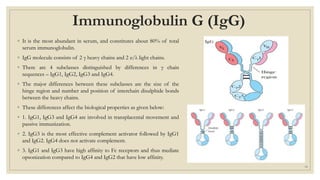
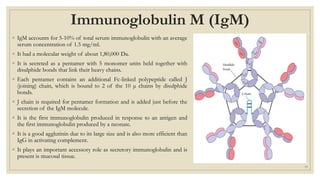
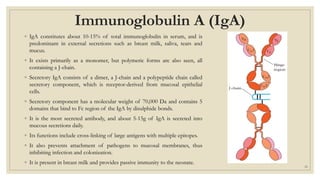
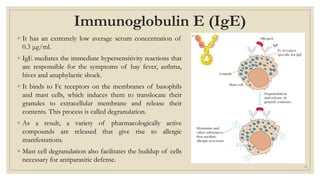
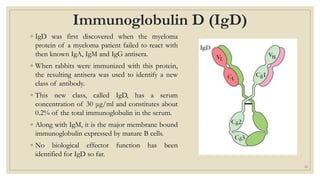
The document summarizes the structure, function, and types of immunoglobulins (antibodies). It discusses the basic four-chain antibody structure consisting of two heavy and two light chains. The five major classes of antibodies - IgG, IgM, IgA, IgE, and IgD - are described along with their structures and functions including antigen binding, opsonization, complement activation, antibody-dependent cytotoxicity, and transcytosis. Electrophoretic studies in the 1930s first identified immunoglobulins in the gamma globulin fraction of serum.