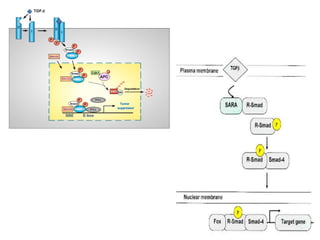
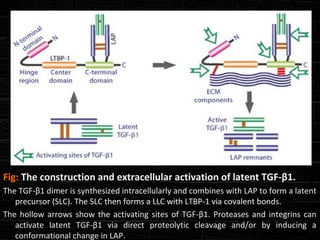
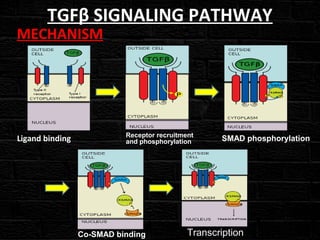
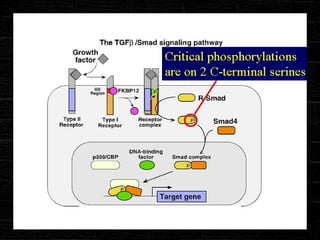
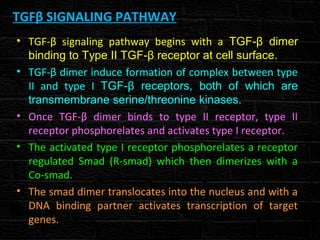
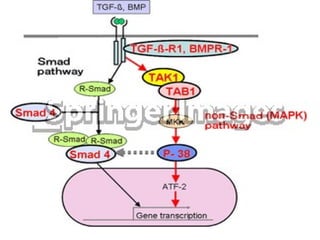
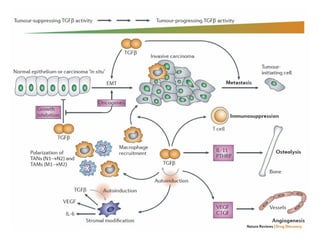
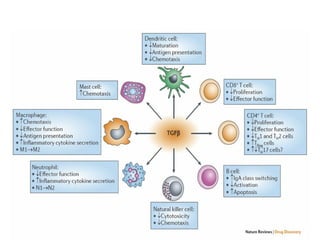
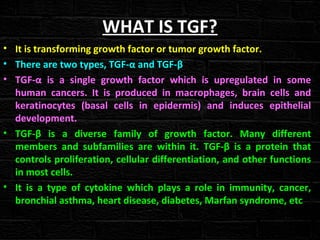
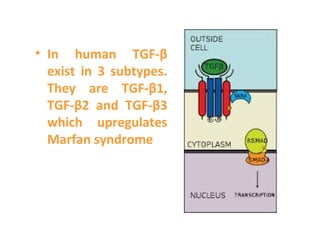
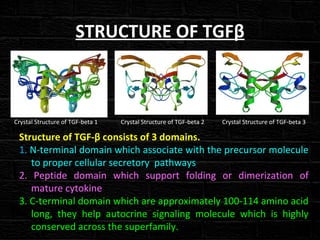
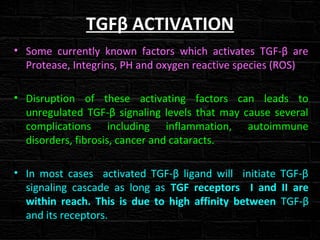
TGFβ is a diverse family of growth factors that controls proliferation and cellular differentiation. TGFβ exists in 3 subtypes - TGFβ1, TGFβ2, and TGFβ3. TGFβ is synthesized as a precursor molecule bound to latency associated peptide (LAP) forming the small latent complex, which then binds to latent TGFβ binding protein forming the large latent complex. The large latent complex is activated via proteases or integrins that cleave LAP, releasing active TGFβ to bind receptors and signal downstream. Upon receptor binding, TGFβ phosphorylates SMAD proteins that regulate transcription of target genes controlling various cellular processes.
![TGFβ Activation continued.....
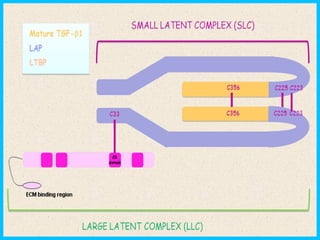
LATENT TGFβ COMPLEX
• TGF-β1, TGF-β2 and TGF-β3 are synthesized as Precursor
molecule which contains propeptide region in addition
to TGF-β homodimer.
• After synthesized TGF-β homodimer interact with
Latency Associated Peptide (LAP). [LAP is a protein
derived from N-terminal region of TGF-β gene product].
• Interaction of TGF-β homodimer with LAP forms Small
Latent Complex (SLC)
• This complex remains in cell unit is bounded by another
protein called Latent TGF-β Binding Protein (LTBP)
forming large complex called Large Latent Complex (LLC)
• It is LLC that get secreted to Extracellular Matrix (ECM)](https://image.slidesharecdn.com/tgfactivationandsignaling-140316064032-phpapp01/85/Tgf-activation-and-signaling-6-320.jpg)