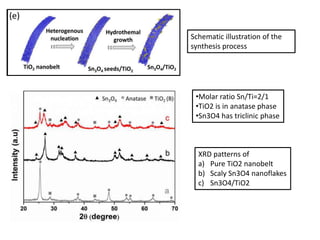
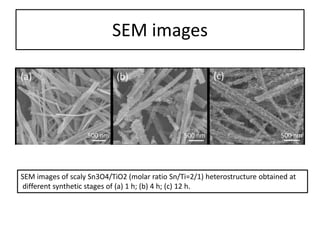
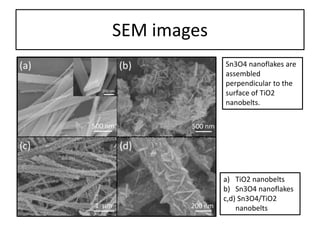
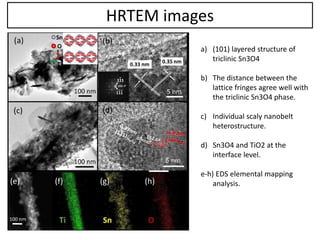
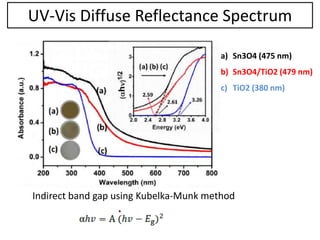
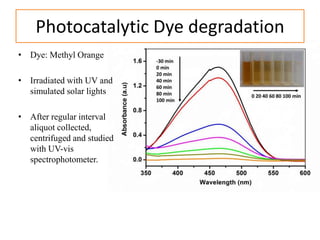
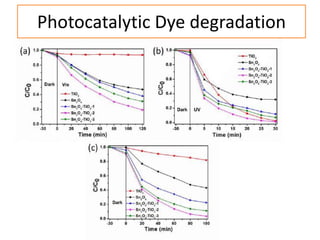
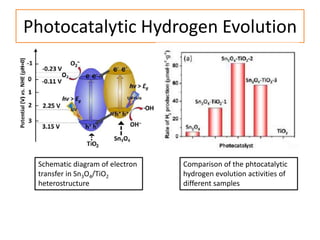
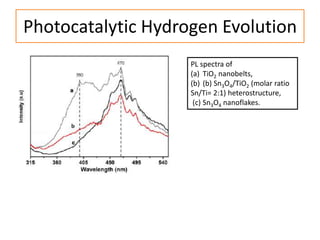
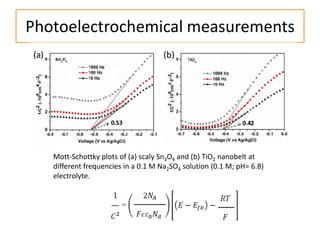
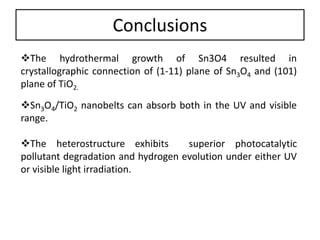
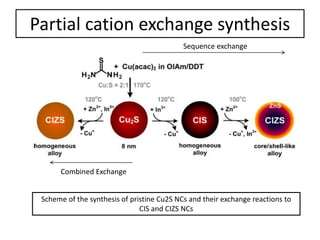
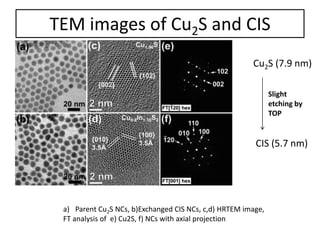
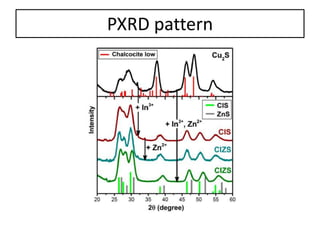
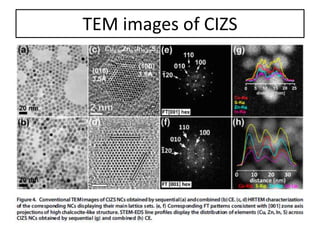
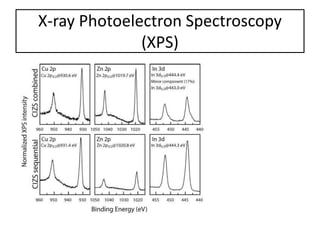
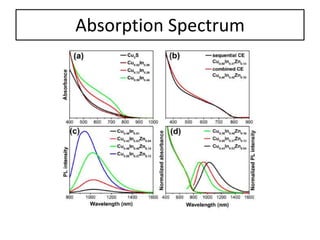
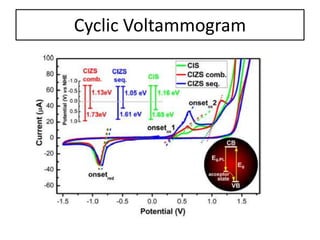
This document summarizes a literature presentation on the synthesis and characterization of Sn3O4/TiO2 nanobelt heterostructures and partial cation exchange synthesis of Cu2S, CIS, and CIZS nanocrystals. Various characterization techniques were used including XRD, SEM, HRTEM, EDS, UV-Vis, PL, and photoelectrochemical measurements. The Sn3O4/TiO2 heterostructures showed superior photocatalytic activity under UV and visible light due to efficient charge separation. The sequential and combined cation exchange syntheses of CIZS nanocrystals resulted in different structures and photoluminescence properties that were tunable based on the Cu:In:Zn ratio.