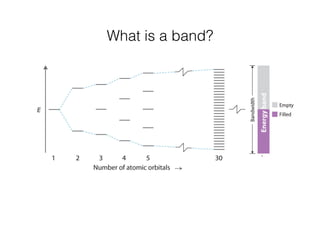
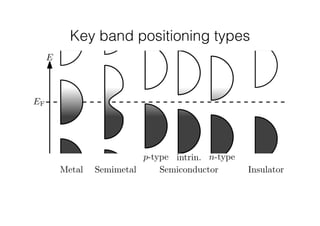
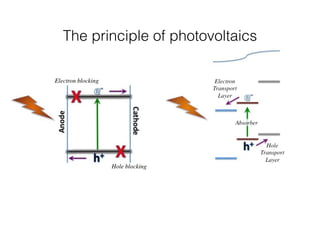
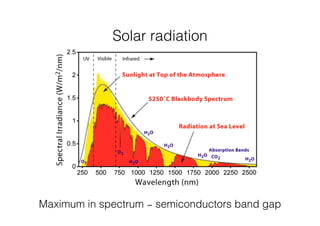
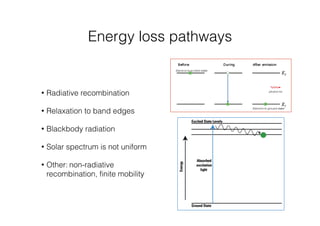
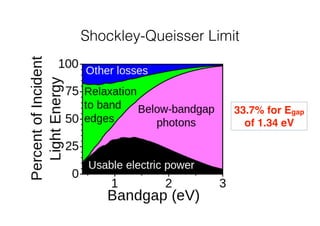
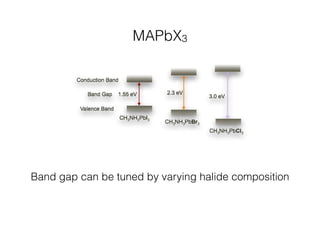
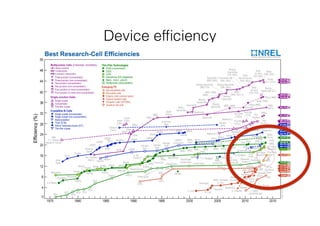
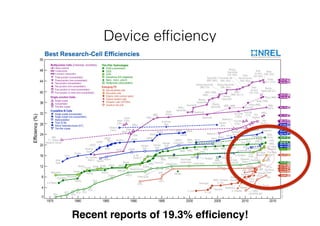
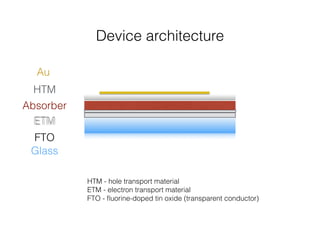
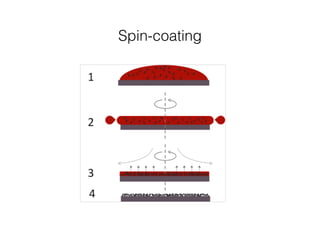
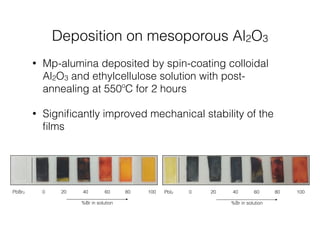
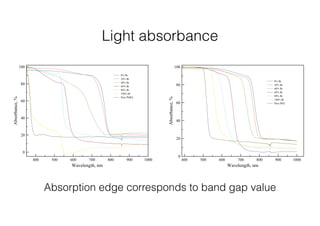
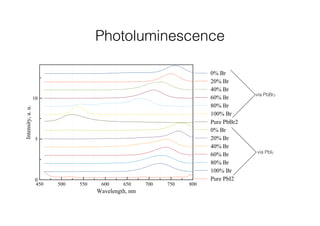
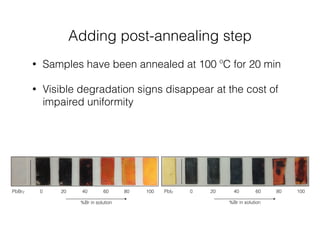
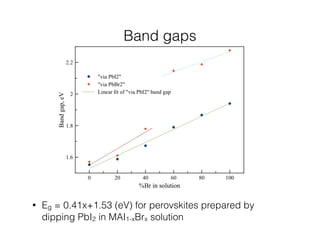
This document discusses band gap engineering of hybrid organic-inorganic lead halide perovskites. It describes how the band gap of methylammonium lead iodide bromide perovskites can be tuned between 1.55-2.29 eV by varying the halide composition in the solution during a two-step deposition process. Using a mesoporous scaffold improves the mechanical stability of the perovskite films. Annealing after deposition prevents instant degradation but affects uniform film formation. The goal is to optimize the fabrication process and characterize the resulting perovskite films.