This document summarizes research on the synthesis and classification of nanostructured materials. It discusses how nanostructured materials can be classified based on their dimensions as zero-dimensional, one-dimensional, two-dimensional, or three-dimensional. Common synthesis methods like hydrothermal/solvothermal and sol-gel techniques are described. The hydrothermal method involves a chemical reaction in a closed system using water or other solvents at high temperature and pressure. The sol-gel method converts a precursor solution into a colloidal solution and gel through hydrolysis and polycondensation reactions. Examples of synthesizing different nanostructured materials like ZnO, Co3O4, ZnS, and TiO2 using these methods are presented from literature.

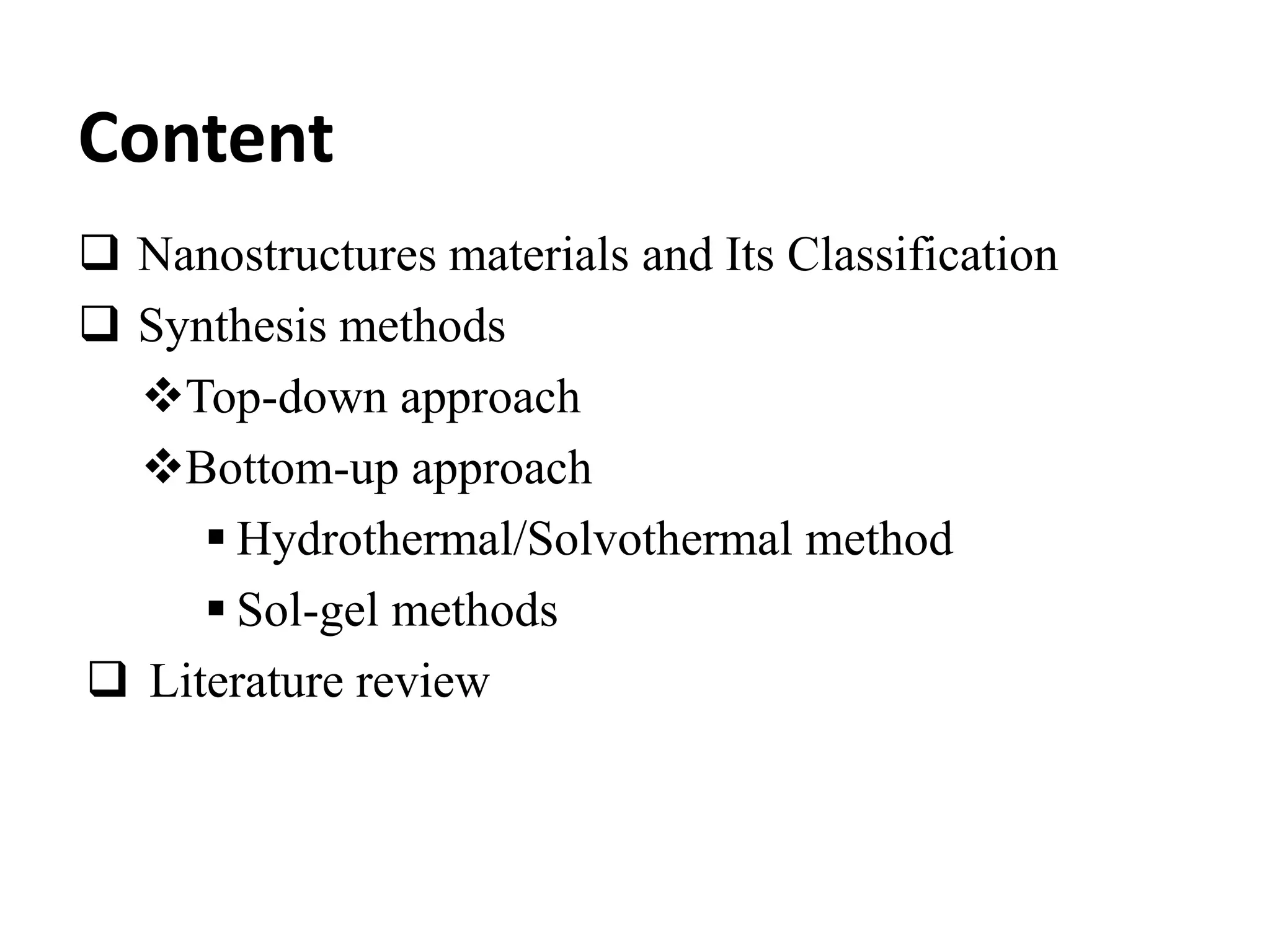


![Yu et al. investigate surfactant's role in tungsten oxide and its hydrates with
different morphologies
Figure 1. SEM image of the synthesized compound under different conditions;
1(a, b). Irregular shape
particles with no
surfactant [50-100nm],
1(c, d). Nanorods structure
in presence of K2SO4
[length-5μm, dimensions-
60-90nm]
1(e, f). Square
shape nanoplates in the
presence of oxalic acid (OA)
[edge length-300nm,
thickness-40nm]
1(g, h). 3D hierarchical
architectures in the presence
of higher OA
Ref: Phys. E Low-dimensional Syst. Nanostructures 79, 127–132 (2016)](https://image.slidesharecdn.com/1ppt-220418110823/75/Synthesis-and-Classification-of-Nanostructured-materials-pptx-6-2048.jpg)


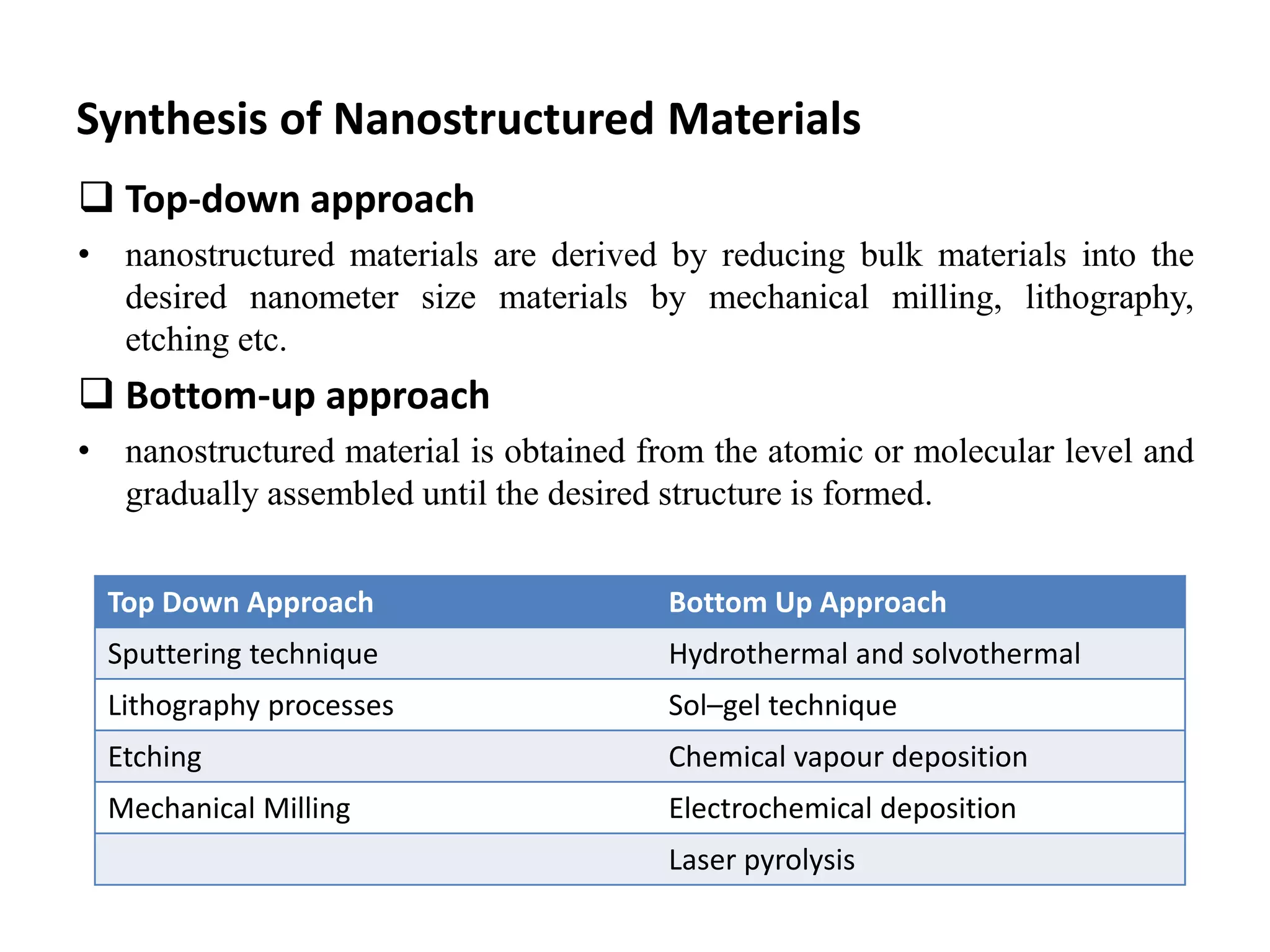
![Literature review based on these techniques
Compound Morphology Raw Materials Conditions Techniques Ref
.
Co3O4 a) Nanobelts
b) Nanoparticles
Co5(OH)6(CO3)2.nH2O, NH3.H2O,
and deionized water
a) 12h at 180℃
b) 12h at 140℃
Hydrothermal/solvot
hermal method
[1]
ZnO a) 3D flower like
b) 2D nanosheets
c) 1D nanorods
d) 0D spherical shape
Zn(NO3)2.6H2O (0.5M) and NaOH
(5M), and distilled water
a) 2h at [125-150]℃
b) 2h at [100-125]℃
c) 5h at 120℃
d) 2h at 150℃
Hydrothermal
method
[2]
ZnS Nanoparticles ZnSO4.5H2O, Na2S.7H2O, and
deionized water
12h at 220℃ Hydrothermal
method
[3]
TiO2 Nanowires TiO2 powder (Degussa P25), NaOH
(15M)
72h at 170℃ Hydrothermal
method
[4]
ZnO Nanoparticles Zn(CH3CO2)2 (Zinc acetate), citric
acid
2days (sol.)
3days dried (for powder
formation)
Sol-gel method [5]
ZnO Nanorods Zinc Acetate Dihydrate
(Zn(CH3COO)2.2H2O), NaOH,
Ethanol (CH2COOH) and distilled
water.
Few hour Sol-gel method [6]](https://image.slidesharecdn.com/1ppt-220418110823/75/Synthesis-and-Classification-of-Nanostructured-materials-pptx-9-2048.jpg)