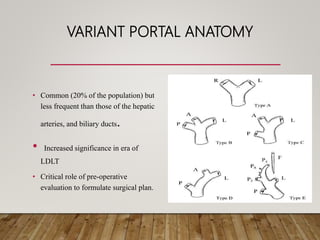
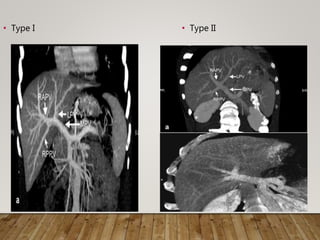
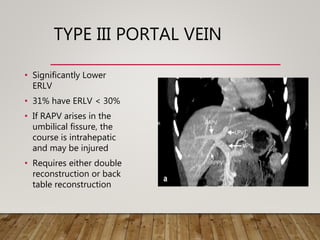
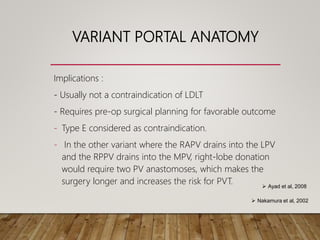
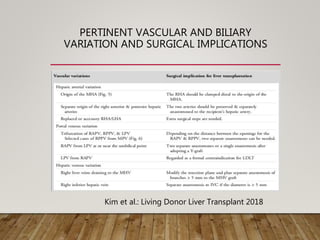
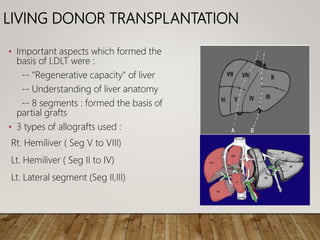
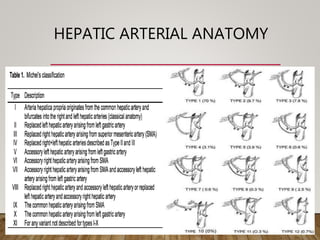
The document summarizes the key steps and considerations in evaluating potential living liver donors. The evaluation involves a multi-stage process including medical history, physical exam, imaging to assess liver volume and anatomy, and further tests as needed. Factors like obesity, steatosis, and variant anatomy require special consideration. The goals are to ensure the donor's safety, obtain an adequate graft for the recipient, and identify any contraindications to donation.










![HEPATIC STEATOSIS
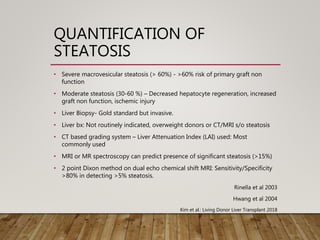
• Imaging studies [US/CT/MRI] can detect the presence of hepatic
steatosis
• Limited in quantifying the degree of steatosis.
• Ryan et al. : could not accurately quantify the degree of hepatic
steatosis using imaging (US or contrast-enhanced CT or both).
Liver Transpl 2002;
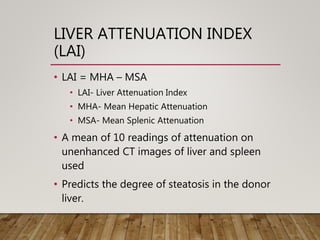
• Iwasaki et al. suggested that quantification of steatosis may be
possible by calculating the liver to spleen ratio (L:S) on
unenhanced CT
• A L:S of ≤1.1 has a sensitivity of 0.83, specificity of 0.82 and accuracy
of 0.82 in detecting steatosis of ≥30%).
Transplantation 2004](https://image.slidesharecdn.com/preopdonorworkupinldlt-181222075907/85/PREOPERATIVE-DONOR-WORKUP-FOR-LDLT-13-320.jpg)









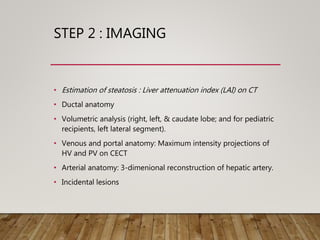
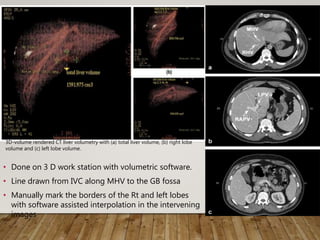
![• Modern CT [e.g., MeVis Liver Analzyler and LiverView, (Bremen, Germany)]
and MRI software produce 3 D liver models that enable
volume measurements (total liver and graft volumes)
permit virtual hepatectomy as part of pre-surgical planning.
• Relatively accurate in estimating actual graft volumes (AGV/AGW)
• Estimated errors: Mainly related to graft perfusion
• Schroeder et al. reported a mean inaccuracy rate for graft volume
prediction of 9 and 12% using CT and MRI, respectively, when compared
to actual graft weight (AGW).
• Salvalaggio et al. found a significant difference between MRI-derived
graft volumes and intraoperatively measured graft volumes.
• Lee et al. found a 9% AGW overestimate by MRI when compared to AGW.
• Sakamoto et al. found that CT inaccuracies ranged from 32%
underestimation to 21% overestimation of AGW by volume.
G. Low et al. Clinical Radiology, 2008](https://image.slidesharecdn.com/preopdonorworkupinldlt-181222075907/85/PREOPERATIVE-DONOR-WORKUP-FOR-LDLT-30-320.jpg)






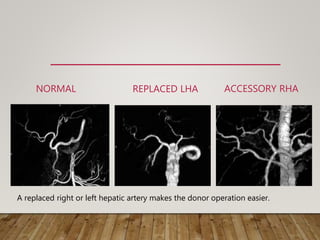
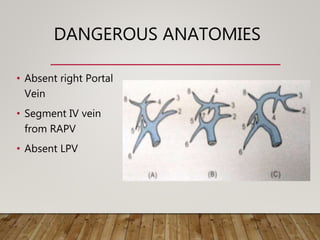
![SEGMENT IV ARTERY
• Variant dominant supply to segment IV by the RHA (11%)
• Needs to be preserved for adequate regeneration in donor
• Significant for both right and left-lobe transplants.
• In right-lobe transplants, if inadvertedly transected – l/t medial
segment ischaemia of the remnant liver.
• Identification of this vessel alerts the surgeon to avoid this
complication by placing the clamp on the RHA distal to the take
off of the segment IV branch.
• Left lobe transplant :Double arterial anatomoses [LHA and
segment IV variant artery] may be necessary
• May be sacrificed if intra hepatic communication with seg II/III
artery present
Sahani D., et al. RadioGraphics 2004
Yamaoka Y.,et al. Transplantation 1994](https://image.slidesharecdn.com/preopdonorworkupinldlt-181222075907/85/PREOPERATIVE-DONOR-WORKUP-FOR-LDLT-49-320.jpg)