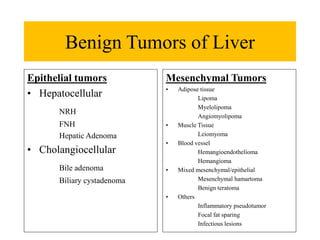
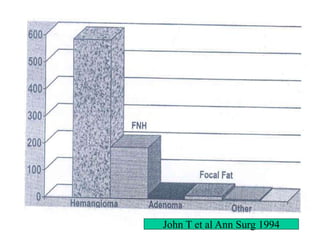
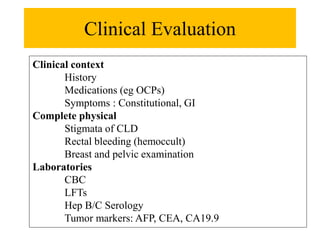
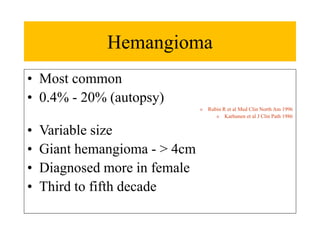
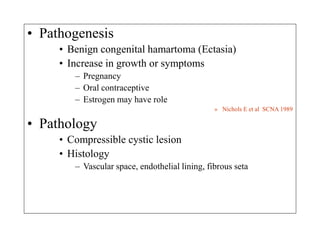
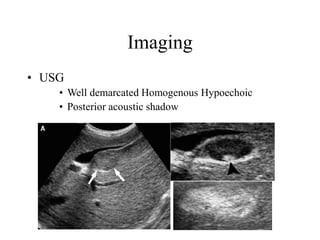
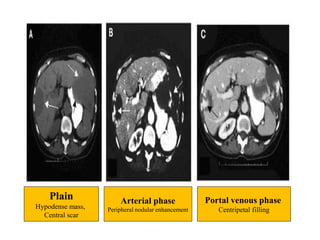
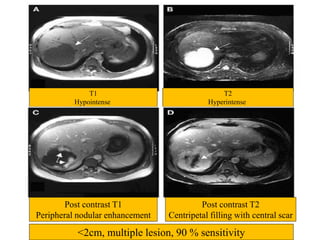
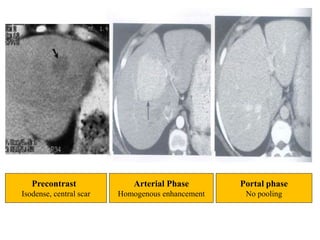
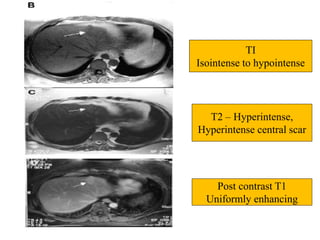
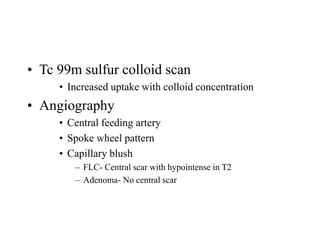
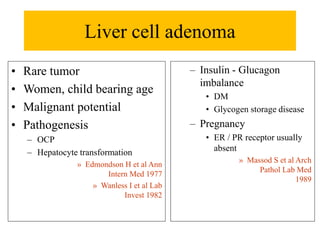
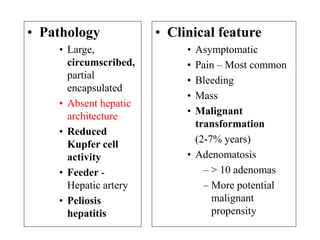
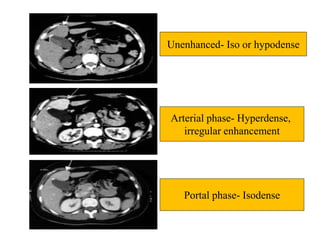
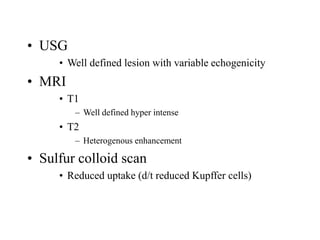
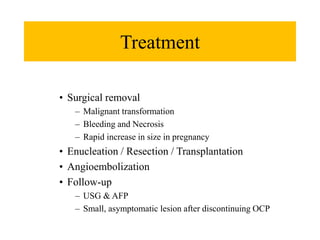
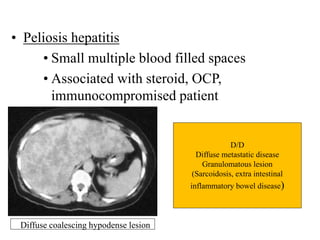
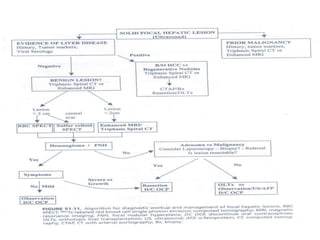
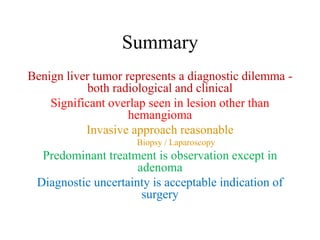
Benign liver tumors present diagnostic challenges due to overlap between lesions on imaging and clinical features. Hemangiomas are typically the only clearly diagnosed tumors without biopsy. Biopsy or laparoscopy are reasonable invasive approaches for diagnostic uncertainty. The predominant treatment is observation, except for adenomas which often require surgery due to malignant potential. Diagnostic uncertainty is an acceptable indication for surgical intervention.