Preformulation involves characterizing the physical and chemical properties of new drug molecules to aid in developing safe and stable dosage forms. It provides direction for choosing the dosage form, excipients, composition and process development. Key factors studied include the drug's physical characteristics like crystallinity, hygroscopicity and solubility, as well as its chemical stability when exposed to conditions like oxidation, hydrolysis and photolysis. Understanding how the drug behaves under various conditions helps ensure the dosage form maintains integrity during storage and use.











![IONIZATION CONSTANT(pKa):- 75% of all drugs are weak base 20% are weak acids and only 5% are non ionic amphoteric or alcohol Henderson-Hasselblach equation:- pH = pKa + log[ionised form]/ [unionised form] ---for acids pH = pKa + log [unionised form] / [ionised form]---for base Uses of this equation To determine pKa. To predict solubility of any pH provided that intrensic solubility(Co) & pKa are known To facilitate the selection of suitable salt forming compounds To predict the solubility and pH properties of the salt](https://image.slidesharecdn.com/physiochemicalfactorsinfluencingformulation-111207030135-phpapp02/85/Physiochemical-factors-influencing-formulaon-12-320.jpg)









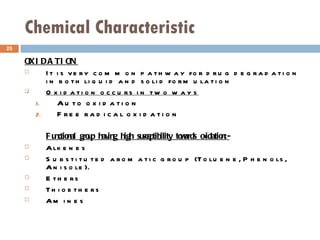
![STABILITY ANALYSIS Development of a drug substance into a suitable dosage form repairs the preformulation stability studies as: [1] Solid state stability [2] Solution state stability Solid state stability :- Solid state reactions are much slower and more difficult to interpret than solution state reactions because of reduced no. of molecular contacts between drugs and excepient molecules and occurrence of multiple reactions](https://image.slidesharecdn.com/physiochemicalfactorsinfluencingformulation-111207030135-phpapp02/85/Physiochemical-factors-influencing-formulaon-22-320.jpg)