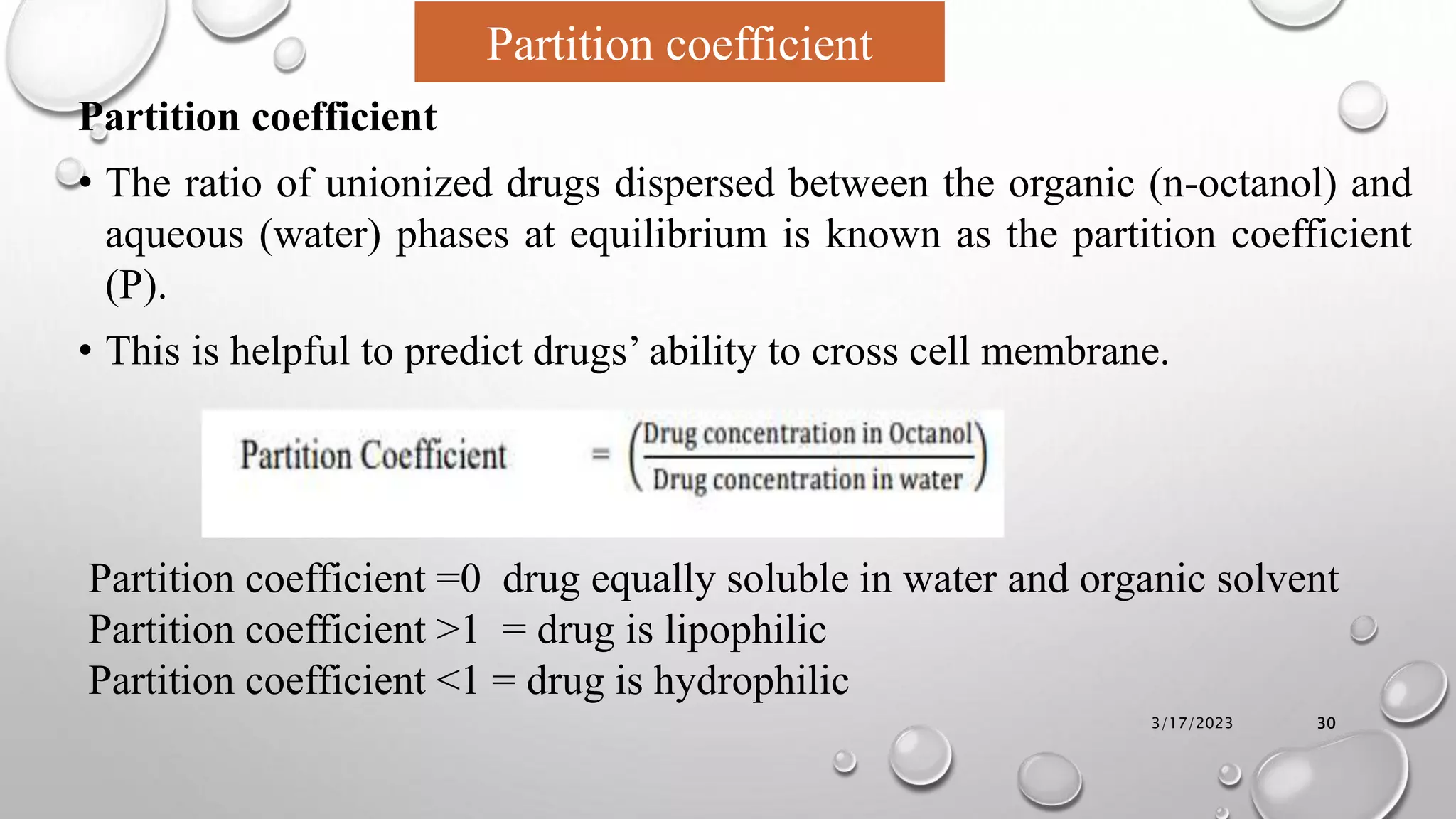
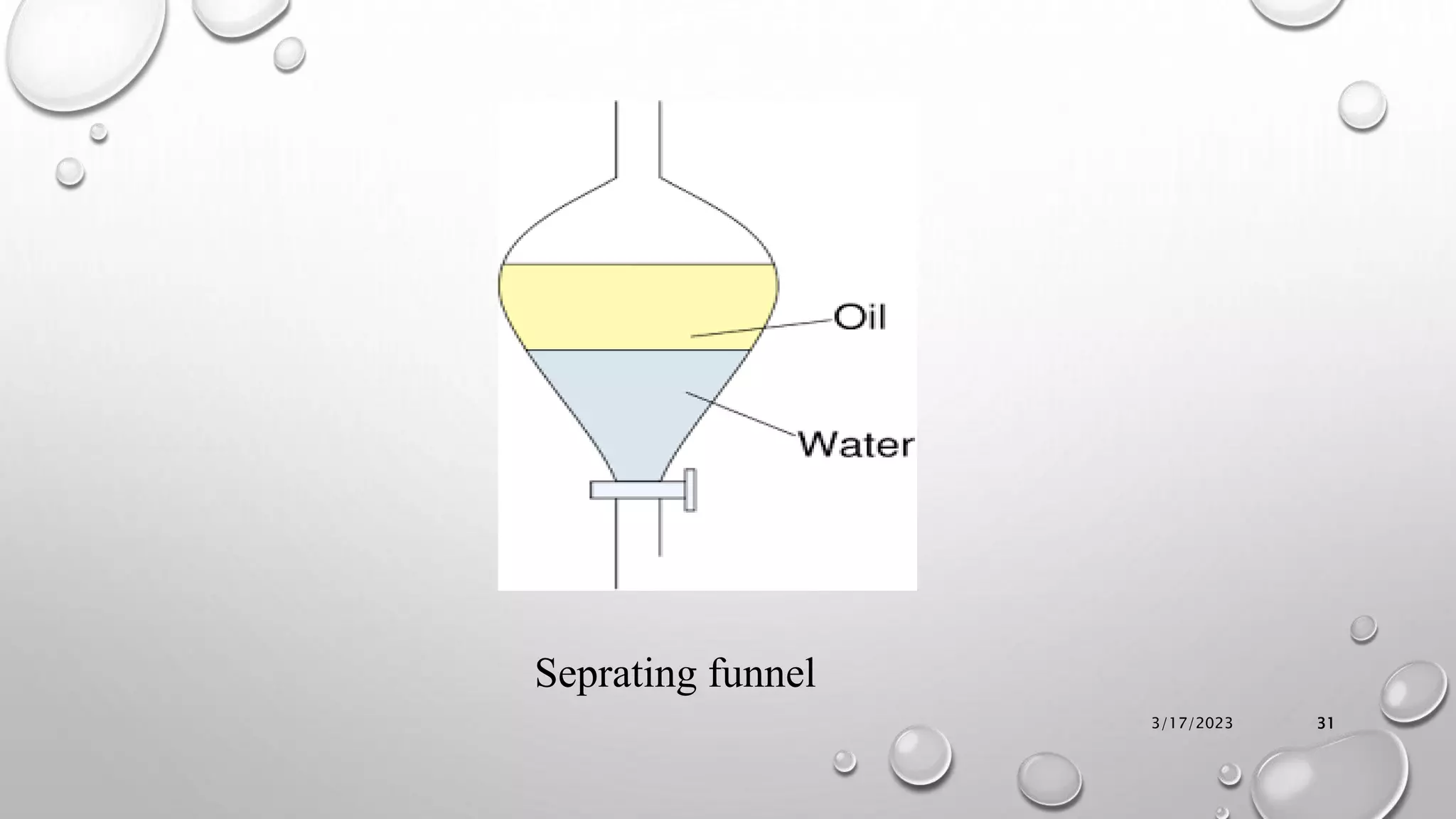
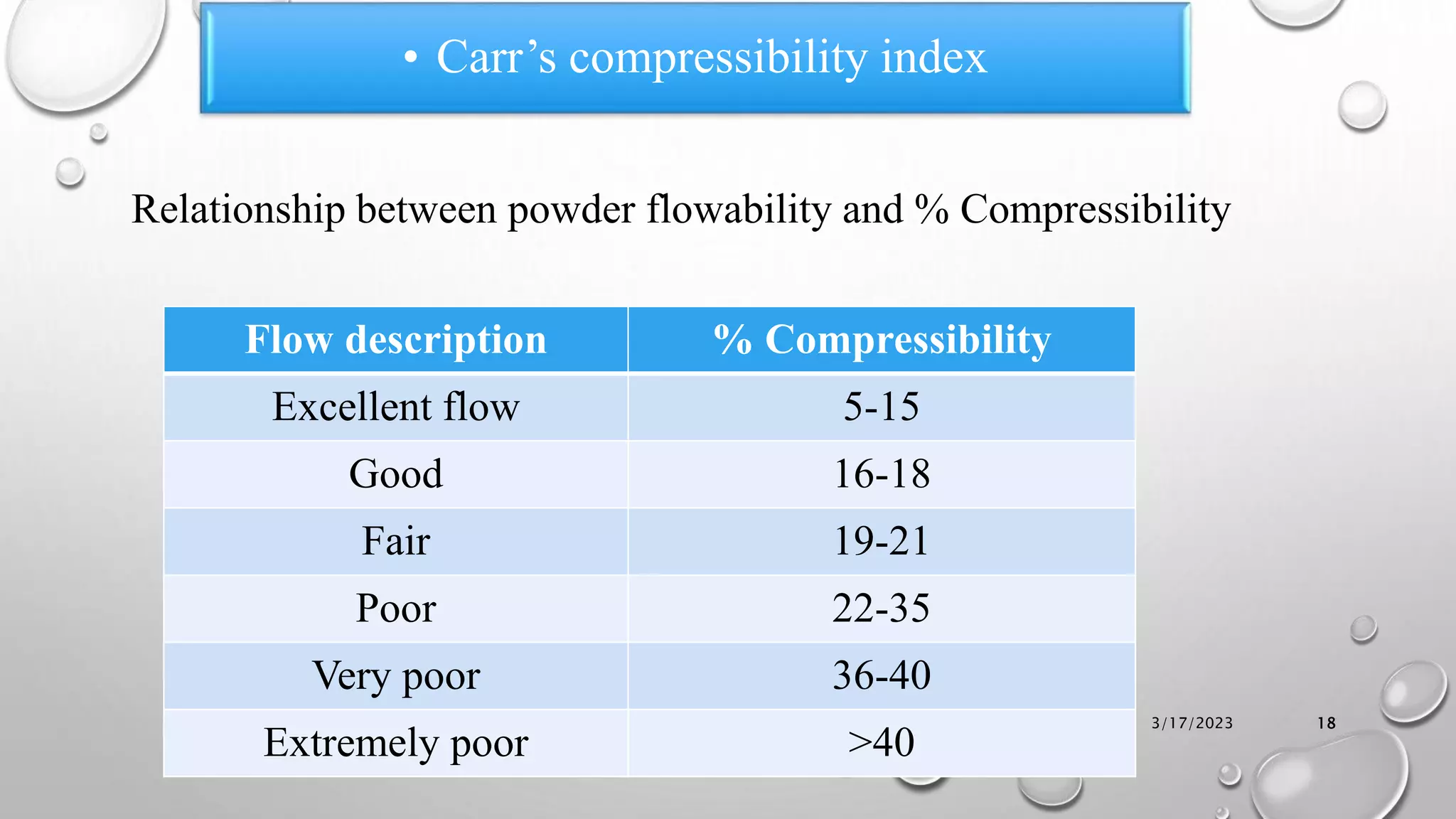
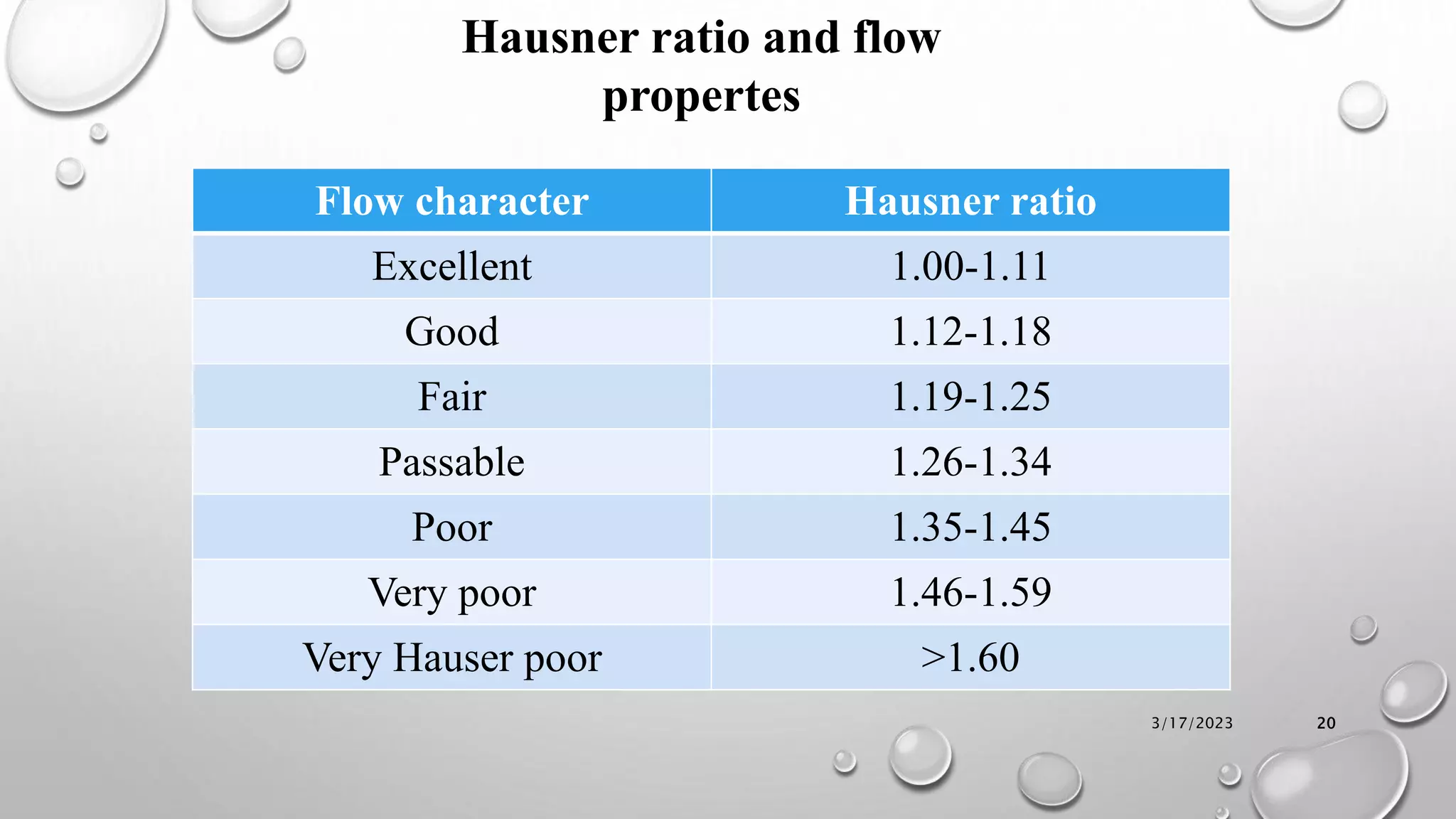
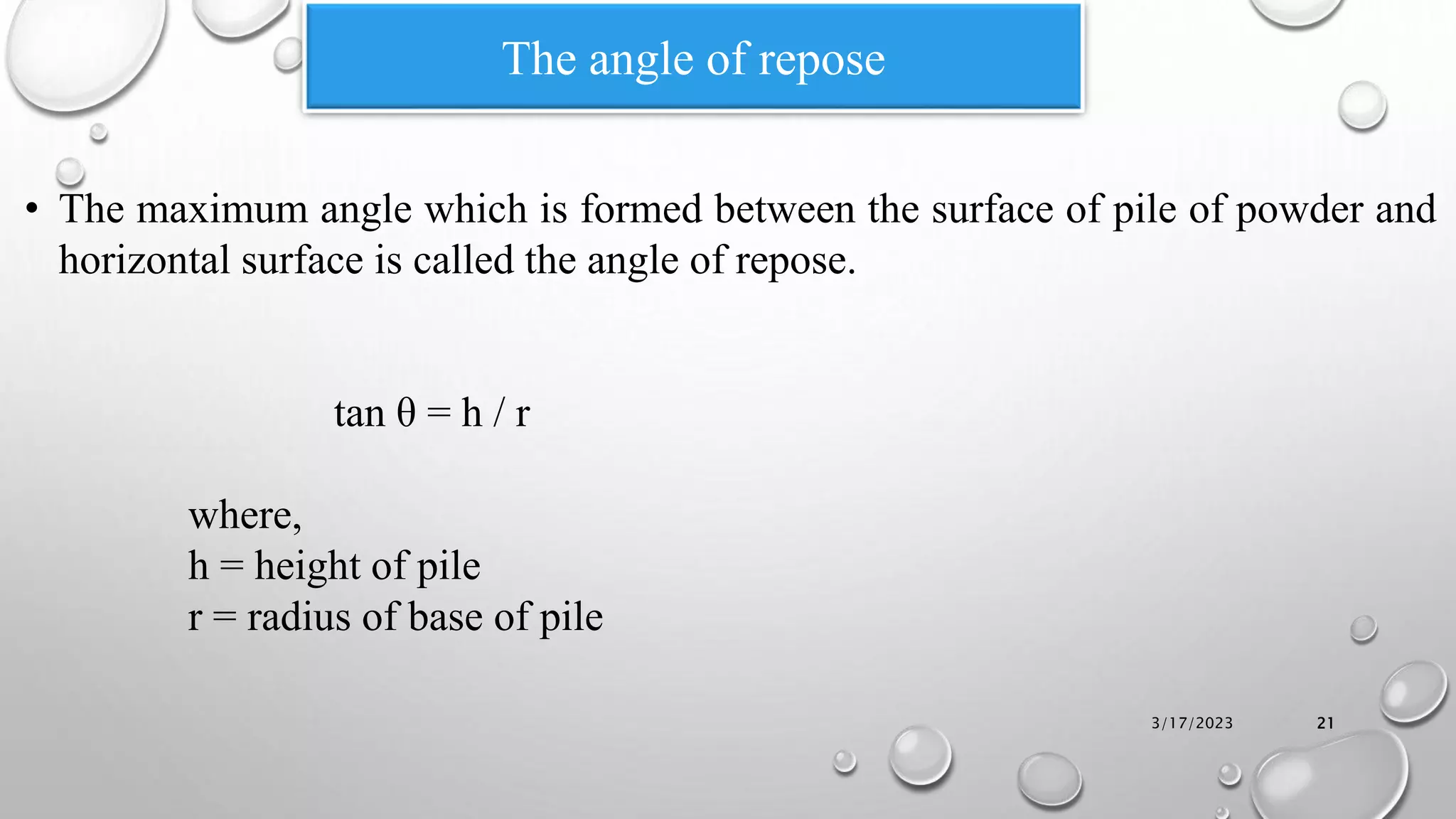
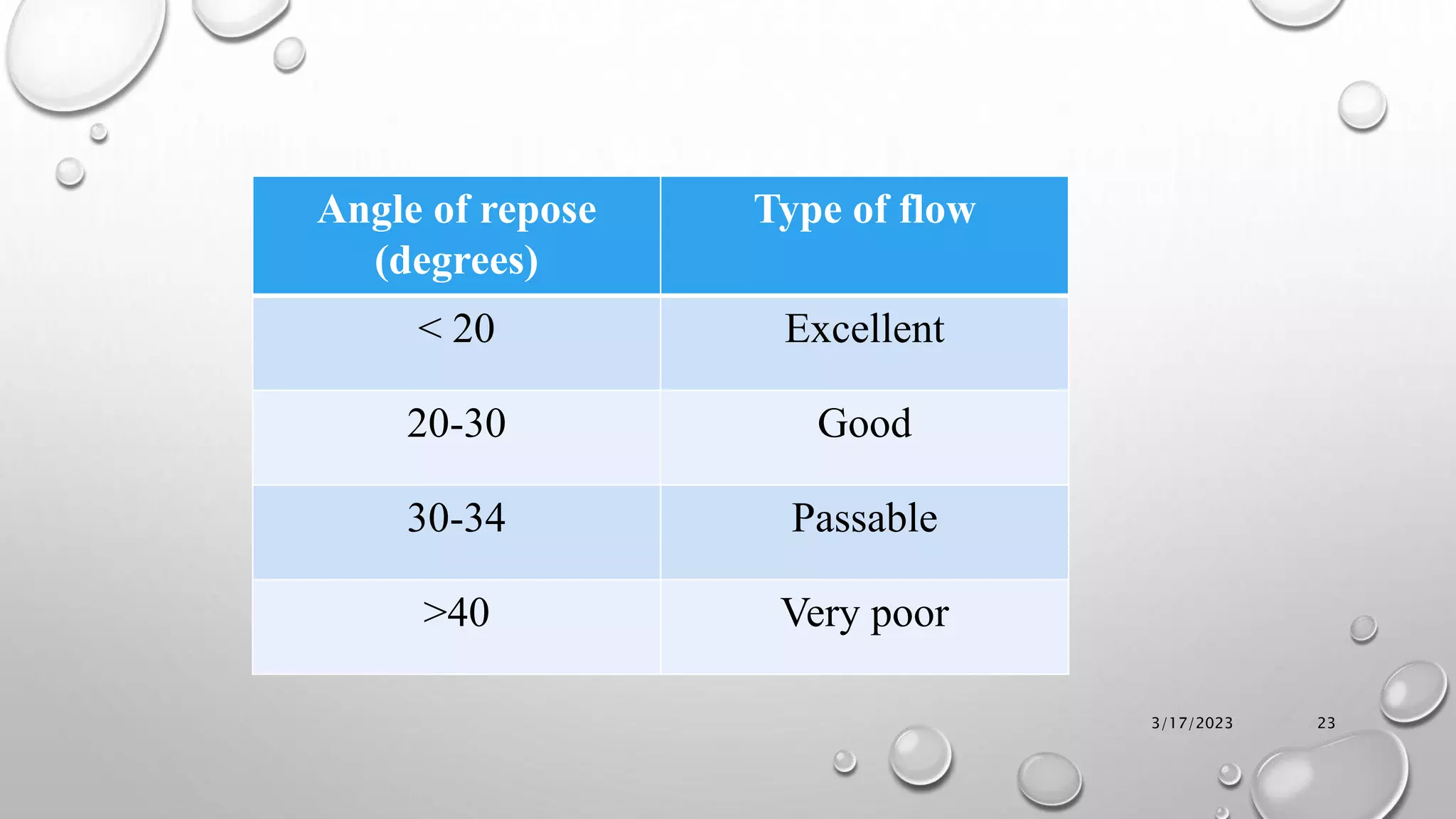
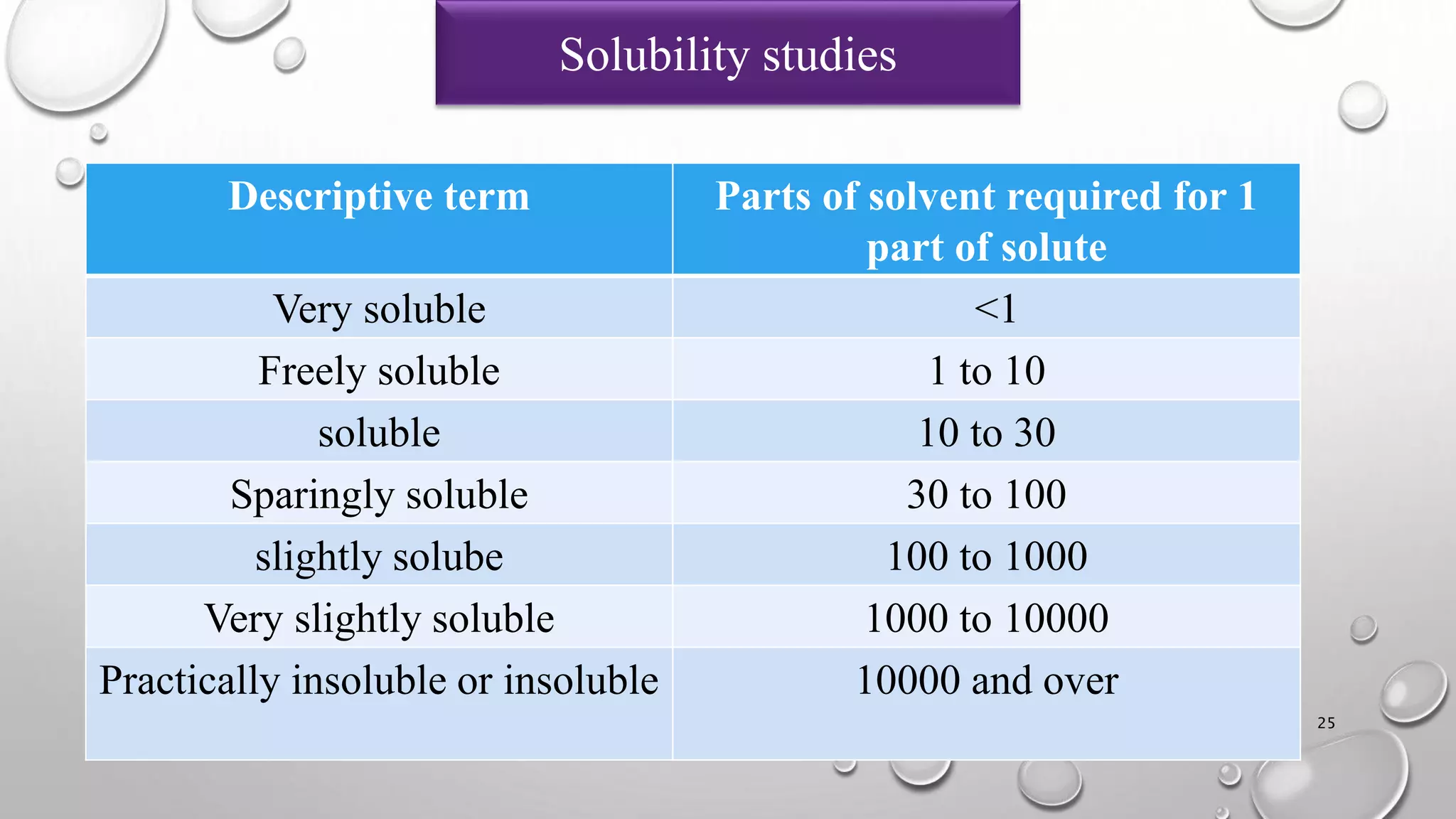
The document summarizes key aspects of preformulation studies. It discusses the objectives of preformulation which are to establish physicochemical parameters, stability, and compatibility with excipients. It then examines various physicochemical properties of drugs including organoleptic characteristics, bulk properties, solubility, crystallinity and polymorphism. Methods for analyzing properties like hygroscopicity, powder flow, and partition coefficient are also outlined. The importance of preformulation in developing stable dosage forms is emphasized.











![• Solubility and absorbtion can altered by changing pH
• The Henderson -Hasselbach equation provides an estimate of the ionized
and unionized durg concentration at a particular pH.
For acidic compounds,
pH = pKa + log (un-ionized drug) / [ionized drug]
for basic compounds,
pH = pKa + log (ionized drug) / [un-ionized drug]
3/17/2023 26
Ionization constant- pKa](https://image.slidesharecdn.com/preformulationpavanbzalte2-230327173613-f9ebb434/75/Preformulation-study-by-Pavan-B-Zalte-26-2048.jpg)