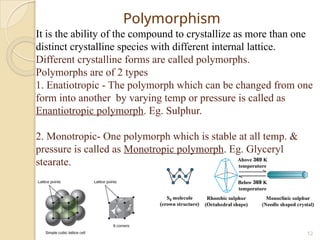
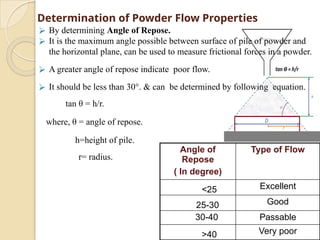
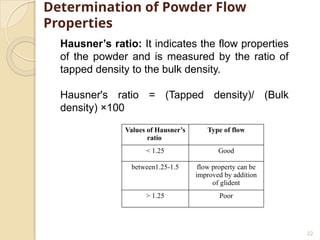
The document discusses preformulation studies essential for the development of stable and effective drug dosage forms by investigating the physicochemical properties of drug substances and their interactions with excipients. It highlights key areas of research such as organoleptic, bulk, solubility, and stability characteristics, alongside methods for assessing particle size, hygroscopicity, and compatibility. The ultimate goal is to ensure a safe and effective delivery system through a thorough understanding of the drug's stability and properties.




















![pKa
determination
24
pKa is the dissociation constant of a drug
The un-ionized drug is lipid soluble thus permeates through lipid
membrane.
The ionized substance is lipid insoluble therefore permeation is slow
Degree of ionization depends on pH
Henderson-Hasselbalch equation
For basic
compounds:
For acidic
compounds:
[ionized]
[un −
ionized]
pH = pKa +
pH = pKa + [un − ionized]
[ionized]
10( pH − pKa )
%ionized =
1 +10( pH − pKa )
Determined by uv spectroscopy, potentiometric titration, titrimetric
method](https://image.slidesharecdn.com/preformulationppt24-25-241022165605-c7965ba7/85/PREFORMULATION-UNIT-I-THIRD-YEAR-B-PHARM-24-320.jpg)












