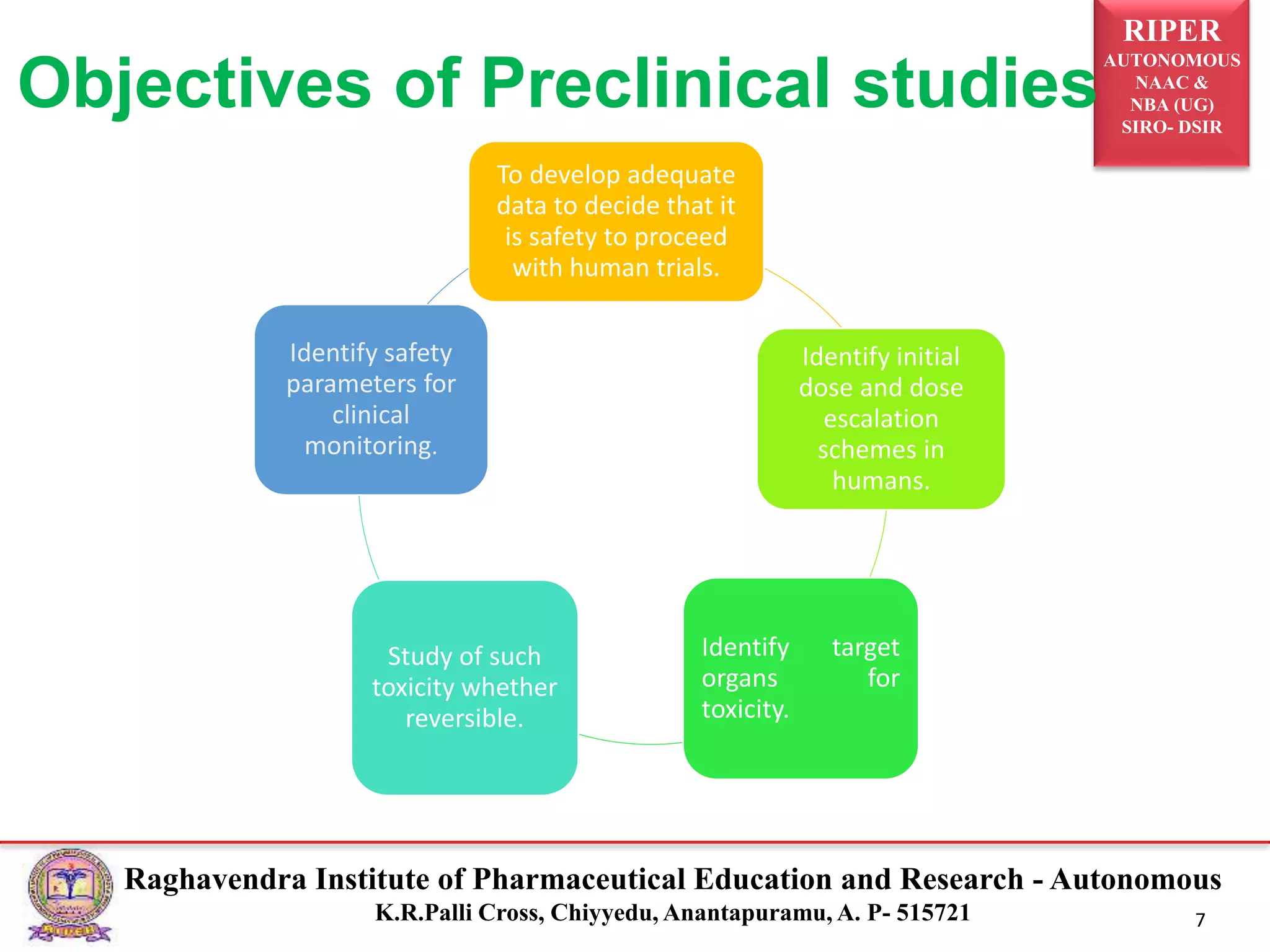
This document outlines the importance and processes involved in preclinical drug development, emphasizing the safety and efficacy assessments conducted using animal models prior to human trials. Key objectives include determining the safety profile, identifying target organs for toxicity, and establishing initial dosing strategies. The document also details various types of preclinical studies and tests to evaluate pharmacodynamic and pharmacokinetic properties.