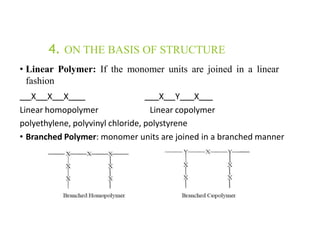
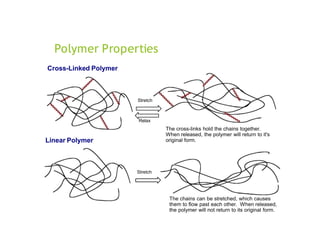
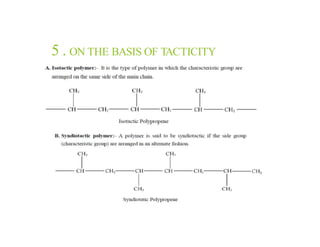
Polymers are large molecules formed by linking together many small molecules called monomers. They can be natural, semi-synthetic, or synthetic. Polymers are classified based on their source, thermal response, mode of formation, structure, tacticity, and application. Common pharmaceutical applications of polymers include use as binders, coatings, and for controlled drug release. Properties like film-forming, thickening, and gelling make polymers useful for various drug delivery applications like tablets, liquids, and controlled release systems. Common polymers used include cellulose derivatives, hydrocolloids, poly(acrylic acid), poly(ethylene glycol), and biodegradable polymers.

![• Copolymer: polymers formed from 2 or more different
monomers
• --[A—B----A----B]---
• Homopolymer: polymers formed from bonding of
identical monomers
• --[A-A-A-A]--](https://image.slidesharecdn.com/pptonpolymers-230922183303-09003ae2/85/PPT-ON-POLYMERS-pptx-3-320.jpg)