The document is a seminar presentation on polymers, covering their definition, characteristics, classification, and applications. It explains that polymers are large molecules formed from repeating units called monomers and categorizes them based on source, structure, and polymerization modes. Applications highlighted include medical uses, consumer products, industrial applications, and sports equipment.
![POLYMERS
Presented by:
Miss. Kamble Akanksha M.
Roll No: 03 [DDS]
First Year M-Pharmacy
Dept. of Pharmaceutics
S.M.B.T.C.O.P, Igatpuri.
SEMINAR PRESENTATION](https://image.slidesharecdn.com/polymers-240107134035-a38542a4/85/Polymers-Ideal-properties-Classification-Types-Applications-1-320.jpg)


![• These are very large molecules consisting of many repeating units
called monomers [small molecules] and are formed by a process
called polymerization.
• In other words, polymers are very large molecules made when 100’s
of monomers join together to form long chains.](https://image.slidesharecdn.com/polymers-240107134035-a38542a4/85/Polymers-Ideal-properties-Classification-Types-Applications-4-320.jpg)



![Sr. No. Type of Polymer Description Examples
1. Linear
Monomers linked linearly to
each other
PE, PVC
2. Branched-chain
Monomers linked like a
branches
Low-density
polyethylene [LDPE]
3. Cross-linked
Monomers are connected to
each other.
Bakelite
II. Based on structure:](https://image.slidesharecdn.com/polymers-240107134035-a38542a4/85/Polymers-Ideal-properties-Classification-Types-Applications-8-320.jpg)
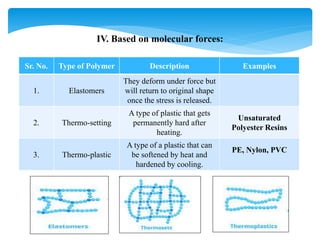
![III. Based on mode of Polymerization:
Sr. No. Type of Polymer Description Examples
1. Homo-Polymer
It is made-up single type of
monomer. Identical bonding
linkages to each monomer
unit.
Polypropene,
Polyethene
2. Co-polymer
When two or more different
monomers together
[different repeating units]
Buna-S](https://image.slidesharecdn.com/polymers-240107134035-a38542a4/85/Polymers-Ideal-properties-Classification-Types-Applications-9-320.jpg)