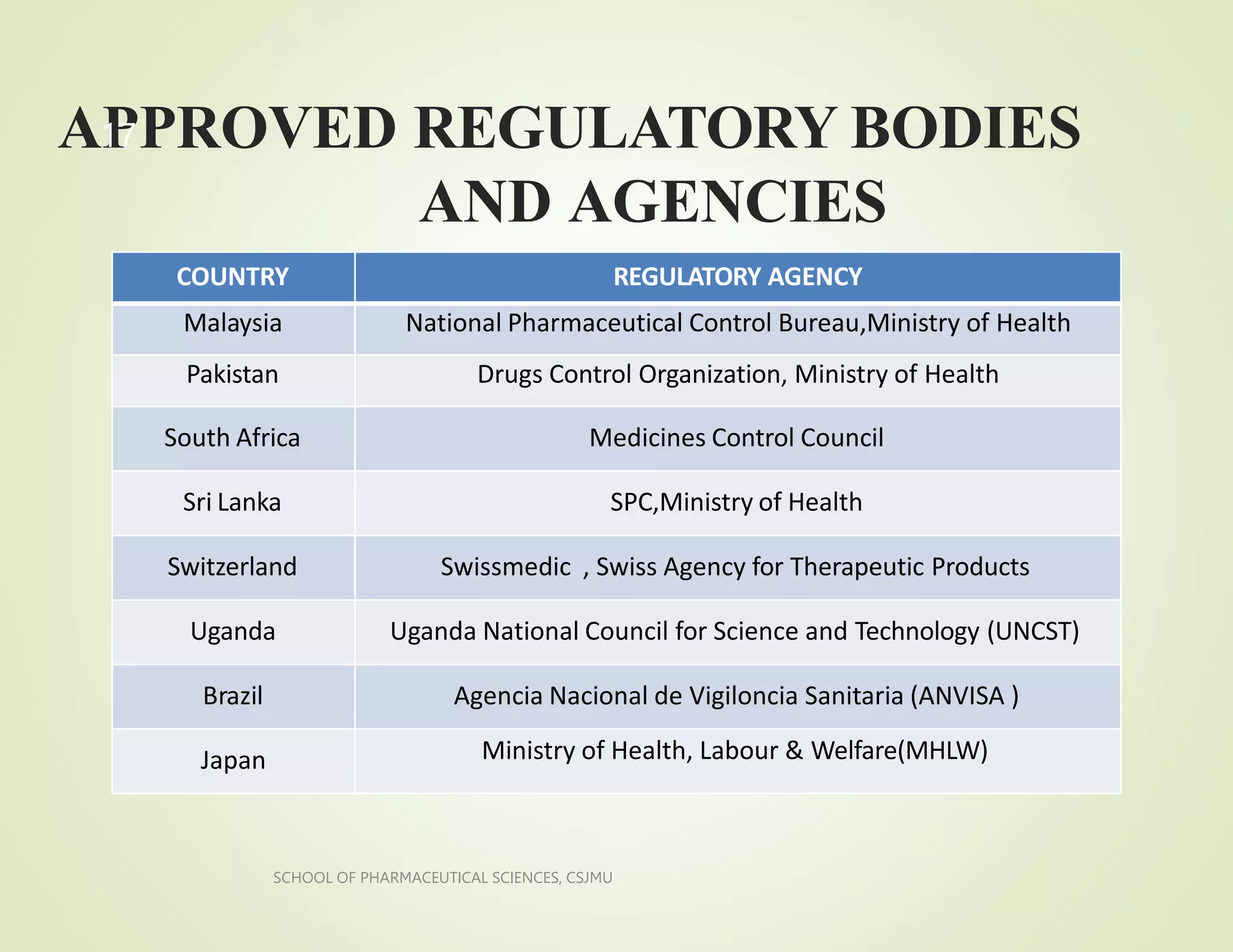
The document provides an overview of regulatory affairs. It discusses the historical development of regulation, including key events that led to increased regulation of drugs and medical products. It also outlines the roles and responsibilities of regulatory affairs departments and professionals in submitting information and ensuring compliance with regulations from agencies like the FDA to obtain approval to market products. The goal of regulatory affairs is to protect public health by ensuring safety, efficacy and quality of drugs and other medical products.