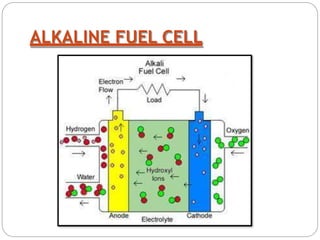
A fuel cell is an electrochemical device that converts chemical energy into electrical energy. It uses hydrogen and oxygen to produce electricity through an electrochemical reaction. There are different types of fuel cells including alkaline, phosphoric acid, molten carbonate, and solid oxide fuel cells which differ in their electrolyte material and operating temperatures. Fuel cells have advantages such as high efficiency, zero emissions, and few moving parts, but are currently more expensive than traditional power sources and require infrastructure to handle and distribute hydrogen fuel. They have applications for power generation, transportation, portable electronics, and more.