This document provides an overview of plant physiology and colloidal solutions. It discusses several key topics:
- Colloidal solutions exist as a colloidal state between true solutions and coarse dispersions due to particle sizes between 0.001 and 0.2 micrometers.
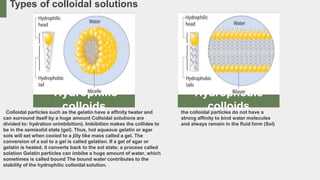
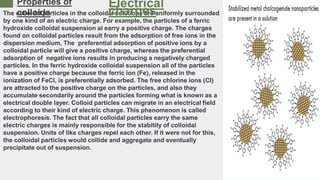
- Preparation methods for colloids include condensation and partitioning. Properties include Brownian movement, the Tyndall effect, osmosis pressure, filtration, adsorption, and electrical charges.
- Colloids can be used in agricultural applications such as the formation of deltas at river mouths and improving soil drainage and aeration through adding limestone or gypsum.