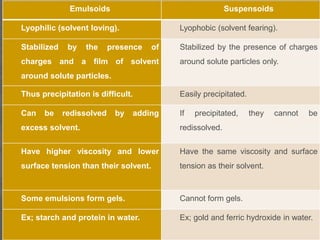
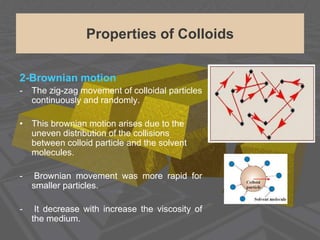
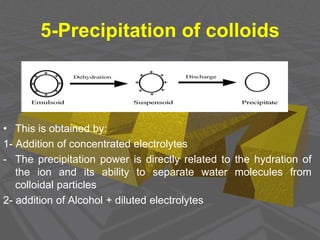
This document discusses physical chemistry concepts related to the states and classification of matter. It provides details on the three states of matter - solid, liquid, and gas. Pure substances can be either elements or compounds, while mixtures contain two or more substances mixed together. The document also defines and compares different types of solutions, including true solutions, colloids, and suspensions. It describes properties of colloids such as the Tyndall effect, Brownian motion, dialysis, ultracentrifugation, and precipitation. Various methods of expressing concentration in solutions are also outlined.