Embed presentation
Download to read offline




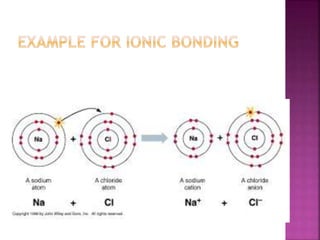






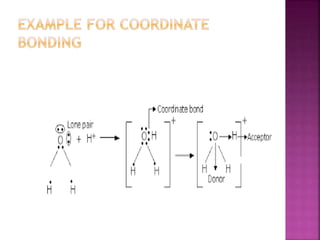
Ionic bonds form when one atom gives up electrons to another atom, resulting in ions of opposite charge attracting one another. Covalent bonds involve the sharing of electron pairs between atoms. Metallic bonds are formed from the attraction between mobile electrons in metals and the fixed positive metallic atoms. A coordinate covalent bond is produced when one atom shares an electron pair with another atom that is lacking a pair.