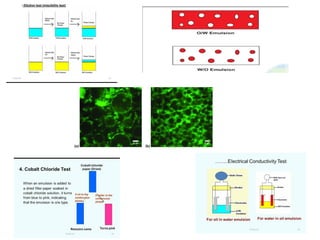
The document provides an overview of gels and emulsions, including their definitions, classifications, preparations, properties, and applications. Gels are semisolid systems created by dispersing small molecules in a liquid medium, while emulsions are heterogeneous mixtures of immiscible liquids stabilized by emulsifying agents. Various types and methods of preparation, as well as the significant roles both substances play in fields such as medicine and food, are discussed.