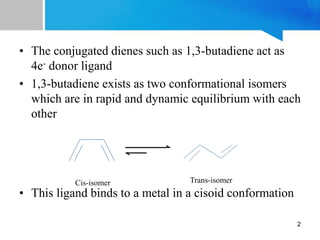
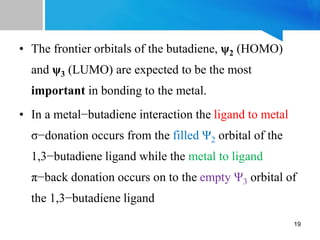
1) 1,3-butadiene acts as a 4e- donor ligand that binds to transition metals. It exists as two conformations and typically binds in a cisoid conformation.
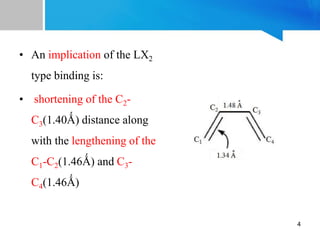
2) Based on the Dewar-Chatt model, 1,3-butadiene can bind as an L2 or LX2 donor type. LX2 binding is more common and results in shortening of the internal C-C bond and lengthening of the terminal C-C bonds.
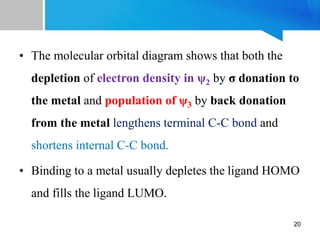
3) Transition metal-butadiene complexes are typically prepared through reactions of metal carbonyls, metal halides, or metal vapors with 1,3-butadiene. The bonding involves donation


![• When a mixture of 1,3-butadiene and Fe(CO)5 is irradiated
by UV light, four CO ligands are replaced by two
molecules of 1,3-butadiene forming a bis(η4-butadiene)
complex
Fe(CO)5 + 2CH2=CH-CH=CH2 [Fe(CO)(η4-C4H6)2]
UV
- 4CO
6](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-6-320.jpg)
![2. Reaction of metal halide with 1,3-butadiene:
Some 1,3-butadiene complexes can be prepared by the
reaction of metal halide with 1,3-butadiene under suitable
conditions
K2[PtCl4] + CH2=CH-CH=CH2 K[PtCl3(η2-C4H6)]
In this complex 1,3-butadiene acts as 2e- donor, dihapto
ligand
- KCl
8](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-8-320.jpg)
![. Metal atom vapour phase synthesis:
The condensation of vapour of a metal in the presence of
1,3-butadiene and suitable co-ligand (such as CO,PR3 etc)
produces butadiene complex
Cr(g) + 4CO(g) + CH2=CH-CH=CH2(g) [Cr(CO)4(η4-C4H6)]
9](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-9-320.jpg)

![[Cr(CO)4(η4-C4H6)]
11](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-11-320.jpg)





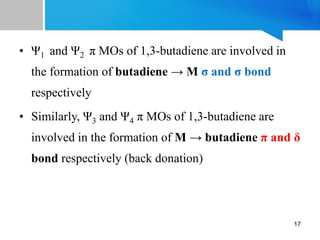
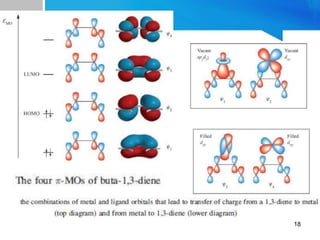





![For ex: [Fe(CO)3(η4-
C4H6)]. Its structure and
bonding is close to (c) in
which π electron cloud of
butadiene is delocalized on
the entire butadiene moiety
[Fe(CO)3(η4-C4H6)]
26](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-26-320.jpg)
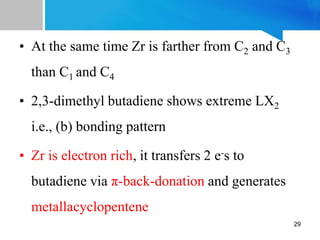
![• If the interaction of ψ3 with metal orbital is important,
the structure and bonding may be represented by (b)
which suggests a shorter C2−C3 distance than two
outer C-C distances i.e., C1−C2 and C3−C4
• Ex: in [Cp2Zr(η4-2,3-Me2-C4H4)] C1-C2 and C3−C4
distances are longer than C2−C3 distance
27](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-27-320.jpg)

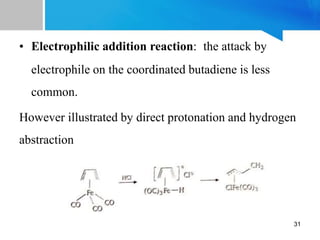
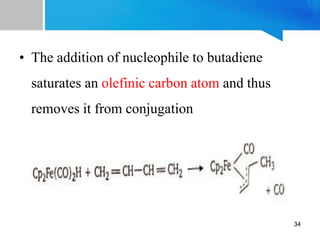
![Properties:
[Fe(CO)3(η4-C4H6)] is a yellow-brown oil which
is soluble in most of the organic solvents
The coordinated butadiene does not undergo
hydrogenation and Diels-Alder reaction
30](https://image.slidesharecdn.com/tm-13-butadienecomplex2023-240410063949-dbe564d9/85/Power-point-presentation-on-TM-1-3-ButaDiene-Complex-30-320.jpg)