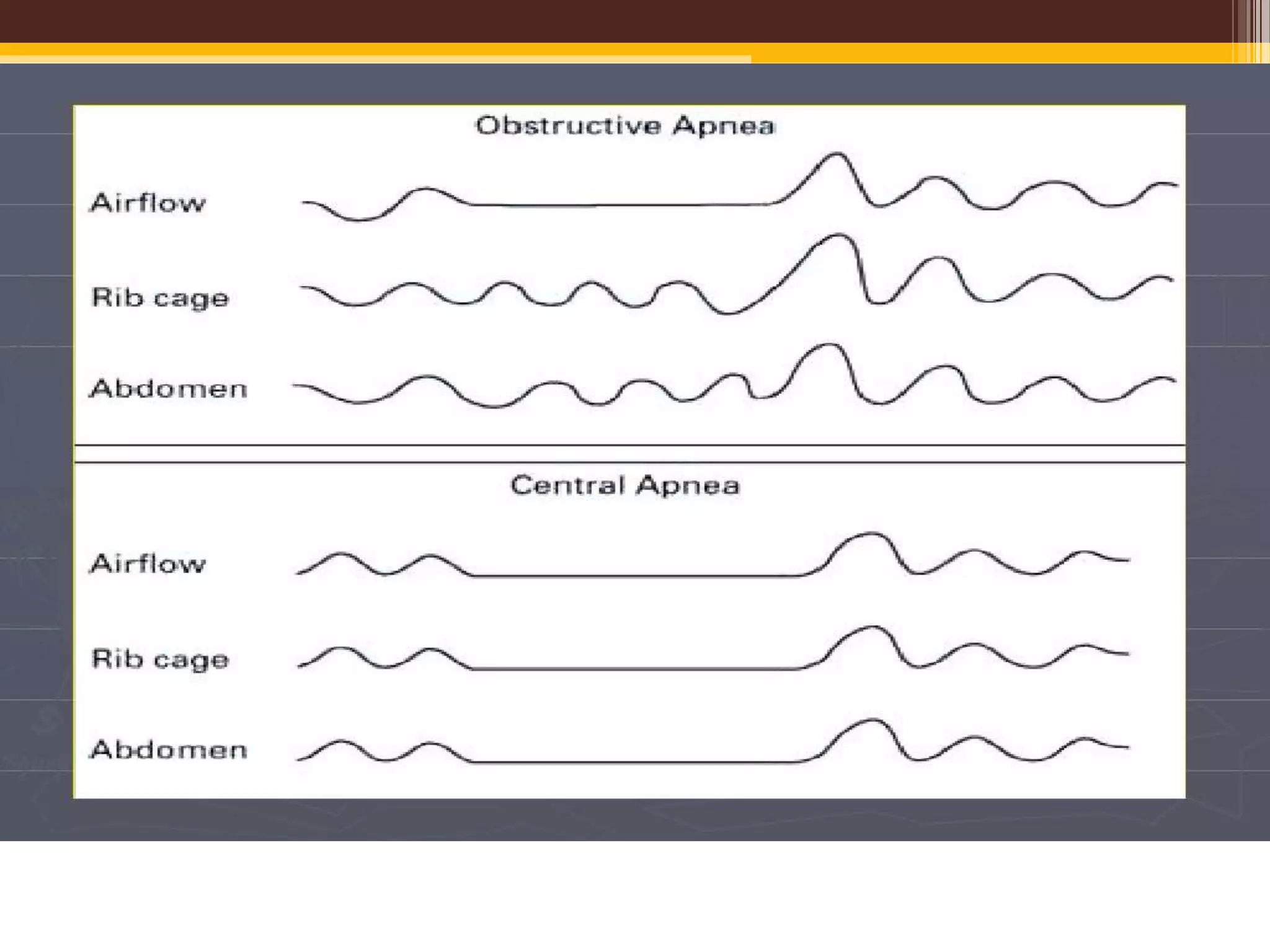
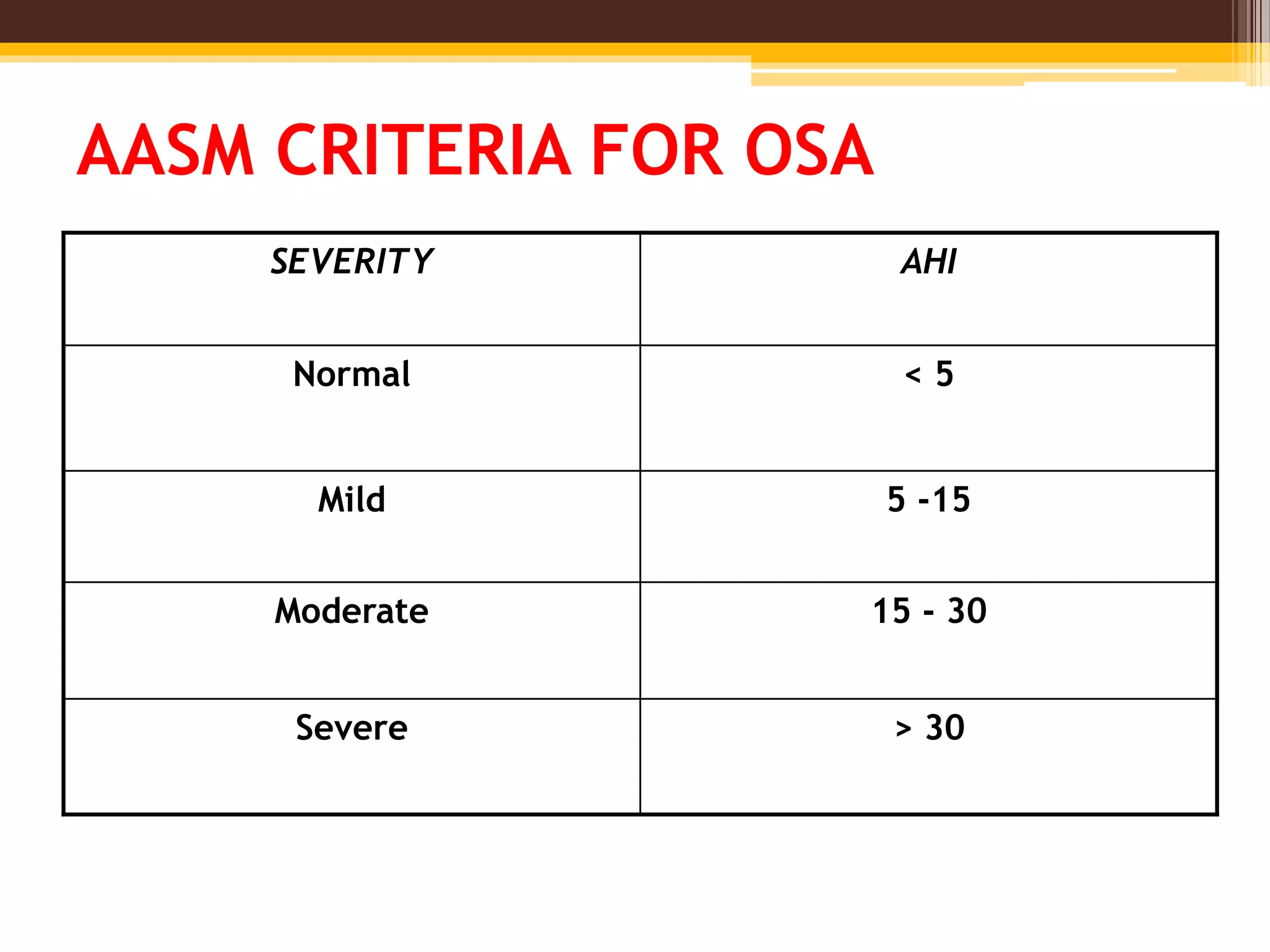
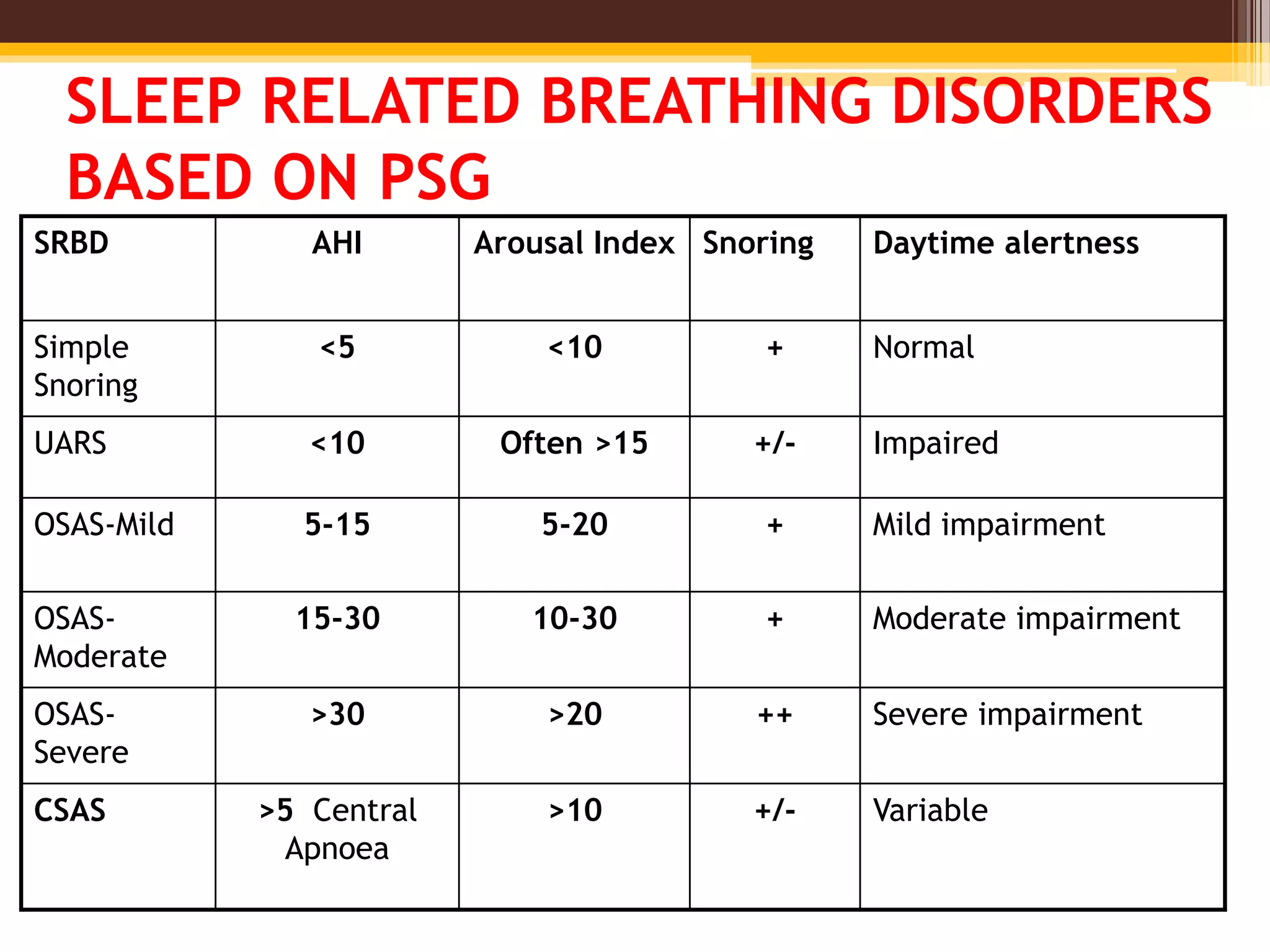
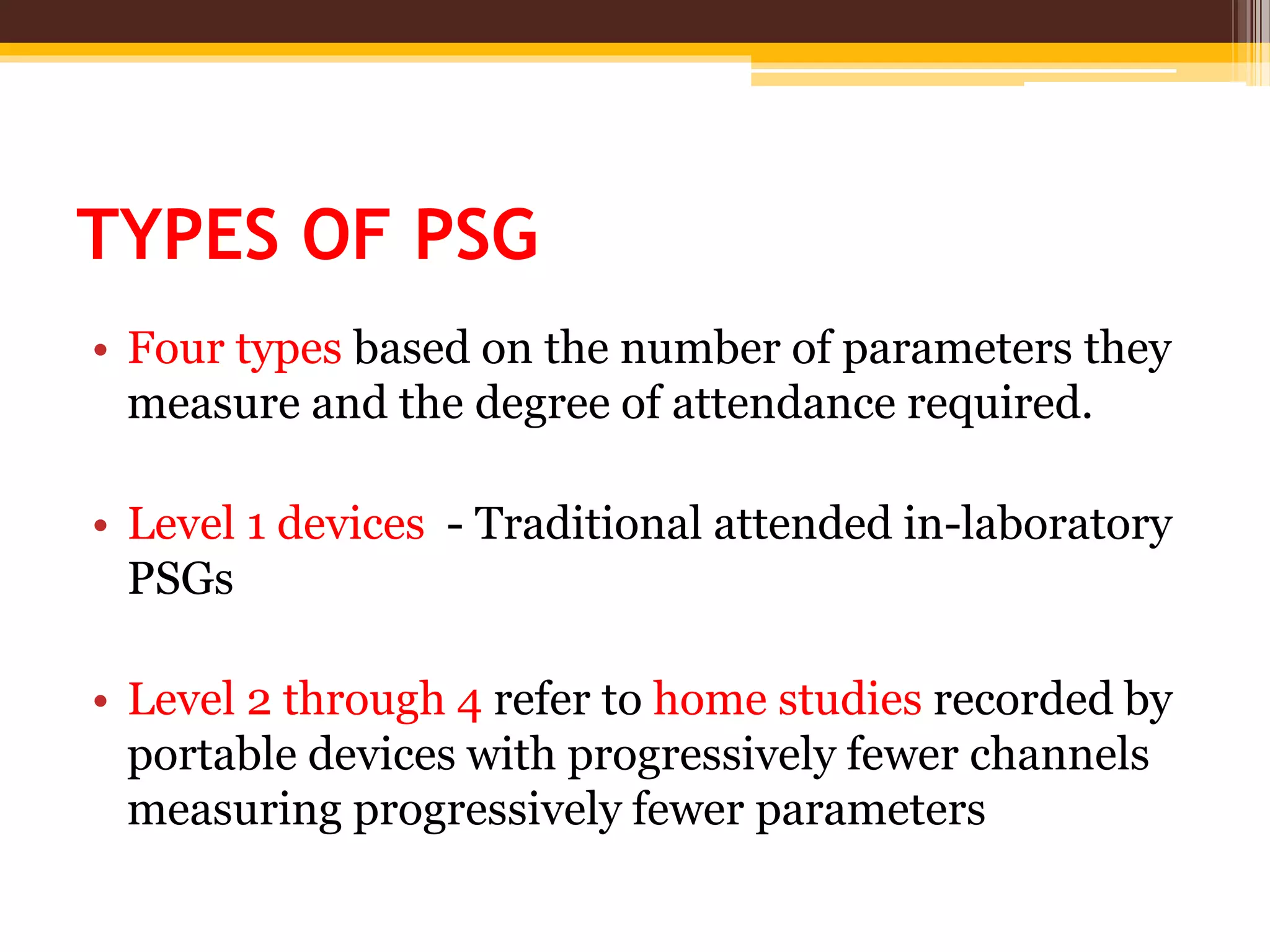
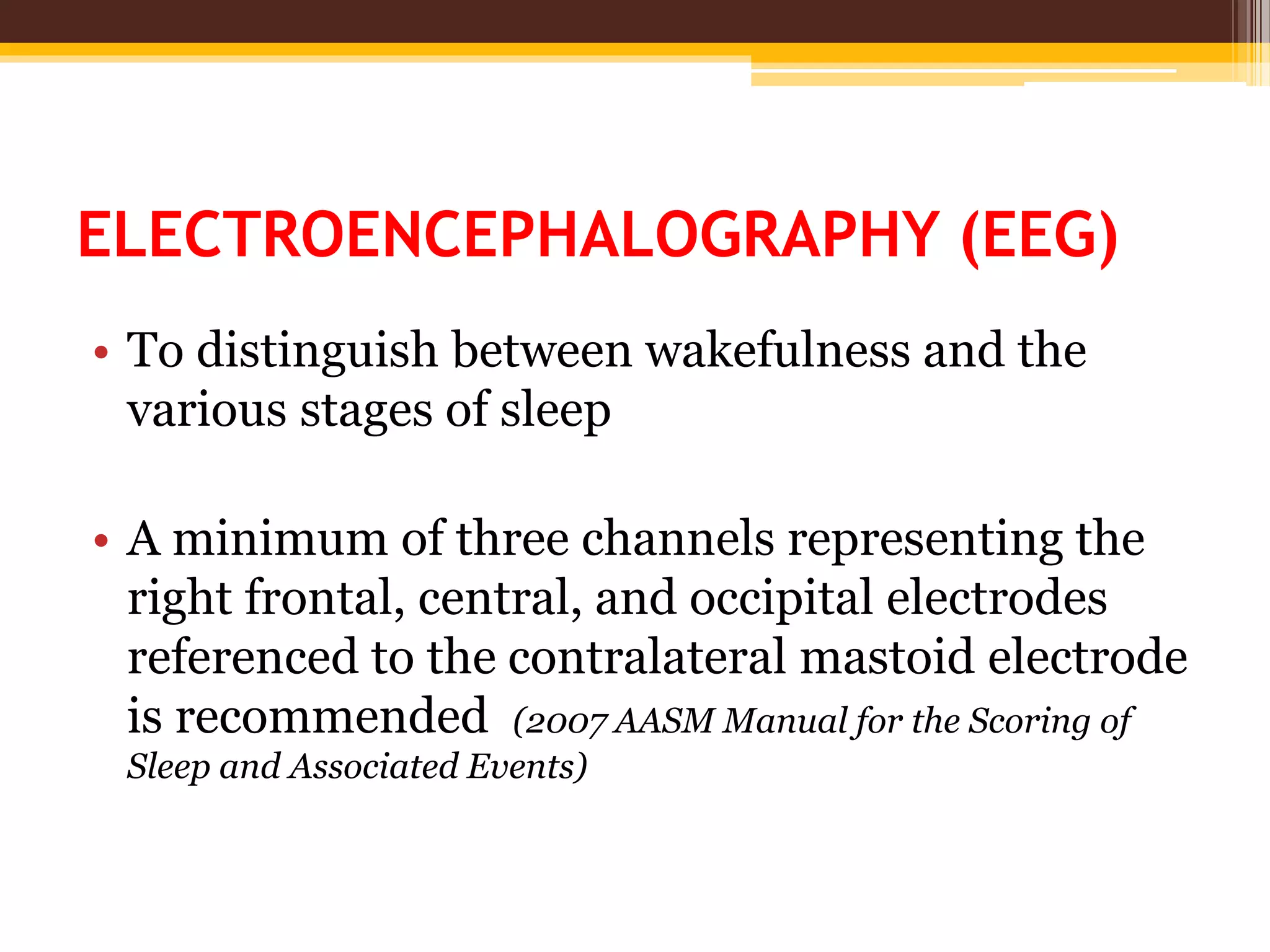
The document provides an overview of polysomnography (PSG), which is a test used to diagnose sleep disorders. PSG involves the simultaneous recording of multiple physiological parameters related to sleep, including brain waves, eye movements, muscle activity, heart rate, respiration, and oxygen levels. It describes the components of a PSG, such as EEG, EOG, EMG, respiratory monitoring, oximetry, and other optional parameters. It also covers patient preparation, test procedures, interpretation of the results, and indices used to diagnose sleep disorders like sleep apnea.






















![INDICES FOR SLEEP APNEA SYNDROMES
• Apnea-hypopnea index (AHI)
The AHI is defined as the average number of
episodes of apnea and hypopnea per hour.
• Respiratory disturbance index (RDI)
Defined as the average number of respiratory
disturbances (obstructive apneas, hypopneas,
and respiratory event–related arousals
[RERAs]) per hour.](https://image.slidesharecdn.com/psgbasics03-04-2017-170613185600/75/Polysomnography-38-2048.jpg)