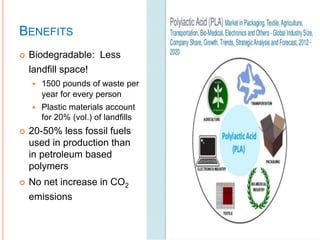
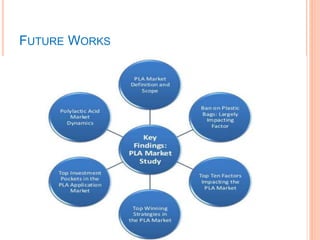
This document discusses polymers and their uses in daily life. It begins by defining polymers as large molecules composed of repeating structural units. Common synthetic polymers mentioned include polyethylene, polypropylene, polyvinyl chloride, and nylon. The document then focuses on poly lactic acid (PLA), describing its production from renewable resources like corn starch, its properties, and its biodegradability. PLA has various applications in agriculture, medicine, packaging and textiles. While PLA production reduces fossil fuel usage, it also releases carbon dioxide and methane during degradation. Overall, the document provides an overview of polymers with a detailed focus on production, properties and uses of the biodegradable polymer PLA.



![ POLY VINYL CHLORIDE
Polyvinyl chloride, more correctly but unusually
poly(vinyl chloride), commonly abbreviated PVC, is the
third-most widely produced synthetic plastic polymer
Density[g/cm3] :1.3–1.45
Chemical formula: (C2H3Cl)n
Melting point :100–260 °C](https://image.slidesharecdn.com/polymersindailylife-160325061928/85/Polymers-in-daily-life-6-320.jpg)