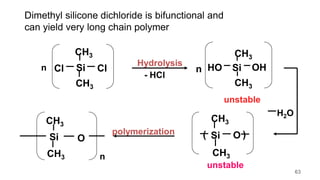
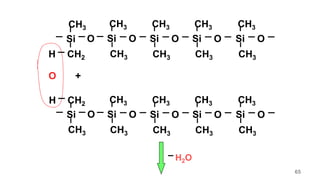
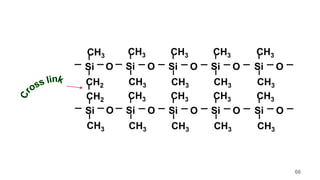
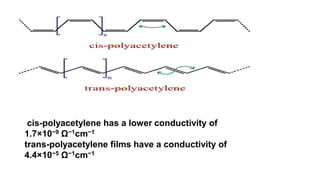
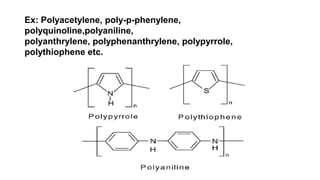
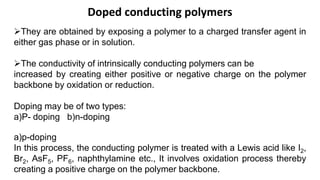
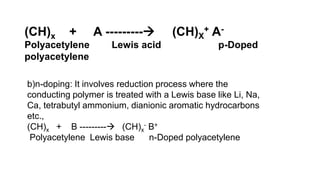
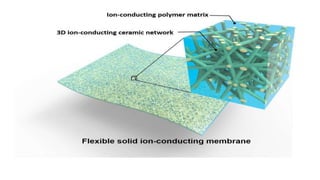
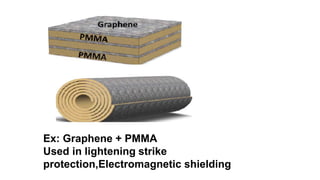
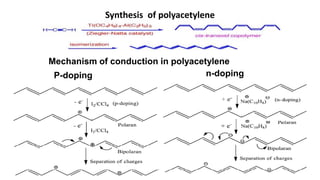
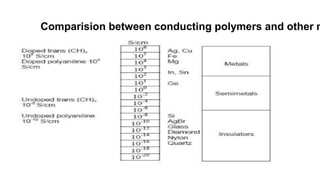
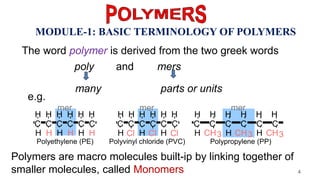
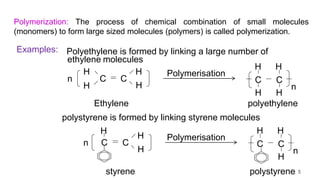
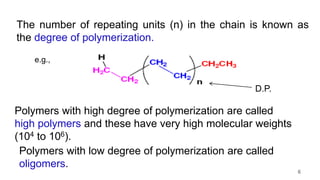
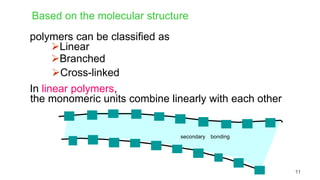
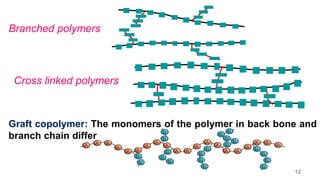
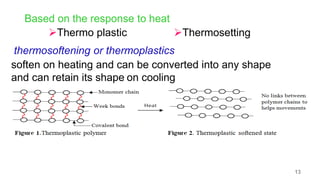
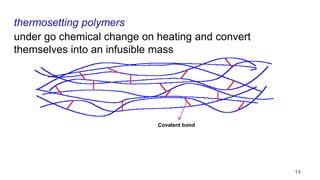
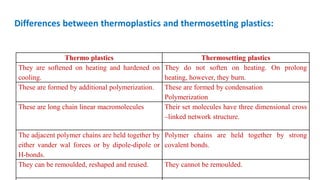
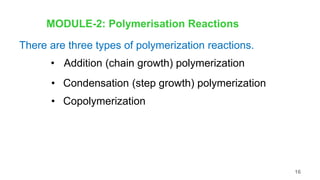
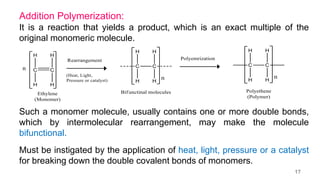
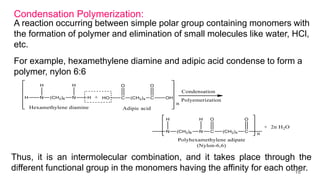
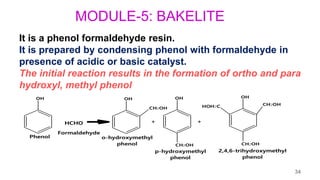
This document provides information on the objectives and outcomes of chemistry courses related to materials chemistry, polymers, and elastomers. The courses aim to help students correlate material properties with internal structure, apply principles of electrochemistry and corrosion prevention, and discuss different types of polymers for engineering applications. Specific topics covered include polymerization reactions, polyvinyl chloride, bakelite, nylon-6,6, Kevlar, and elastomers. After completing the courses, students will be able to analyze and apply concepts related to batteries, water treatment, corrosion prevention, and different types of polymers and their uses.









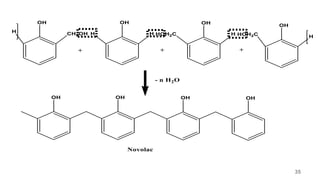
![During moulding, hexamethylene tetramine [(CH2)6N4] are added.
It provides formaldehyde, which converts the soluble and fusible
novolac into a hard, infusible and insoluble solid of cross-linked
structure.
36](https://image.slidesharecdn.com/unit-iiipolymers-230205170055-d78b690b/85/Unit-III-polymers-pptx-36-320.jpg)