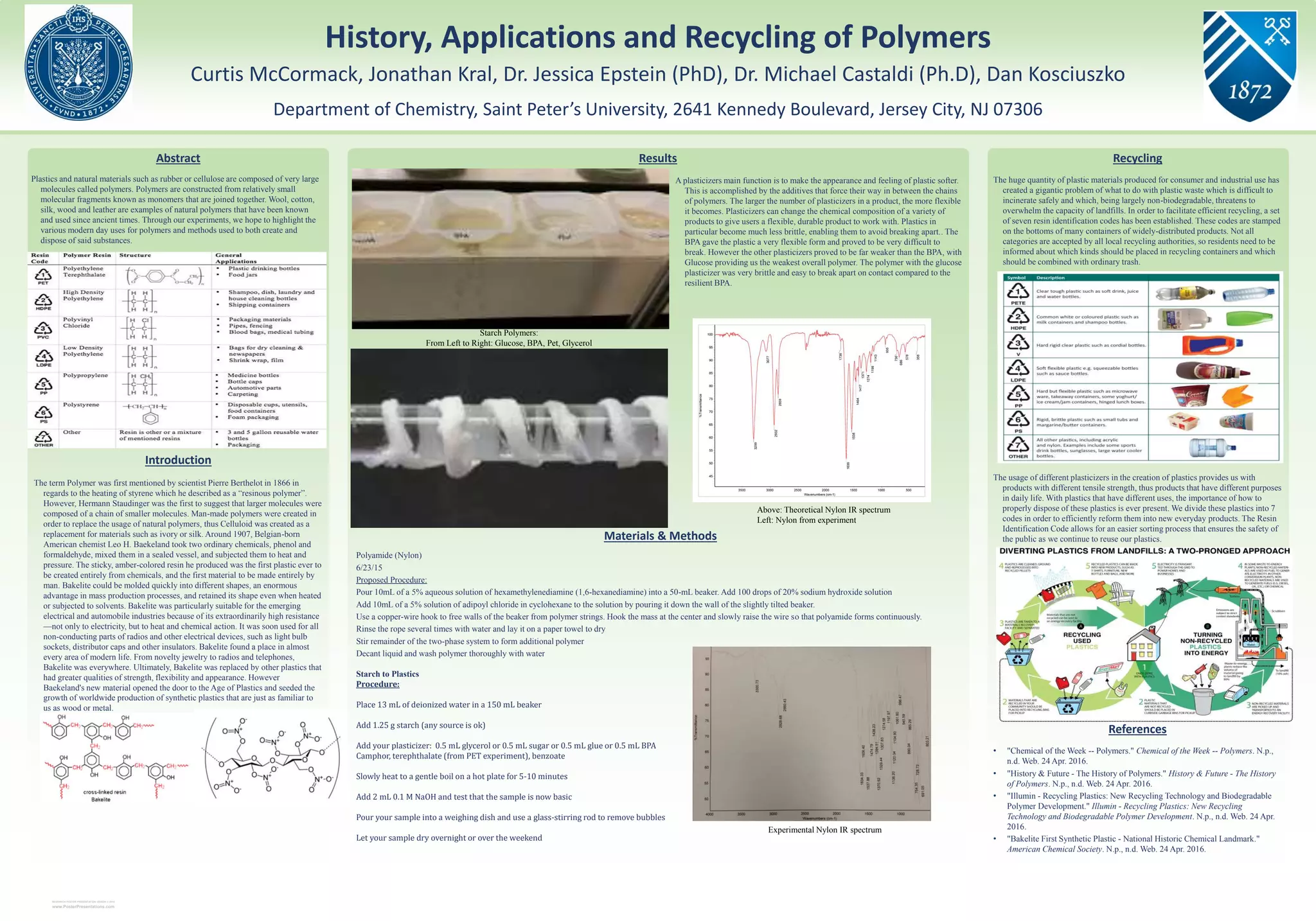
This research poster presentation summarizes key information about polymers, including their history, modern uses, and methods for creation and disposal. Polymers are composed of repeating molecular units called monomers that are linked together into large molecules. Some notable developments discussed include the first synthetic plastic Bakelite created by Leo Baekeland and the establishment of resin identification codes to facilitate plastic recycling. The poster also outlines experiments conducted with various plastics and plasticizers.