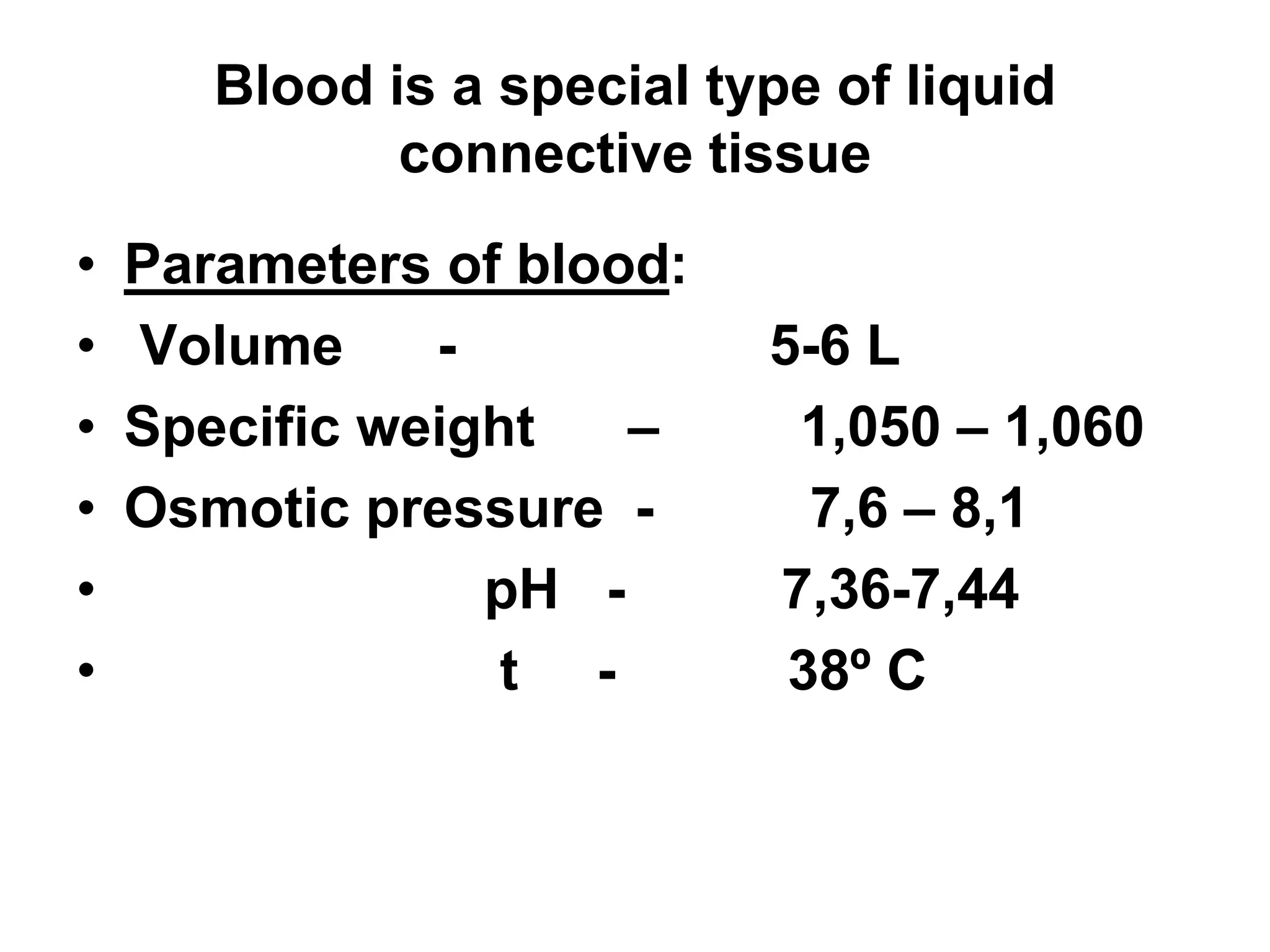
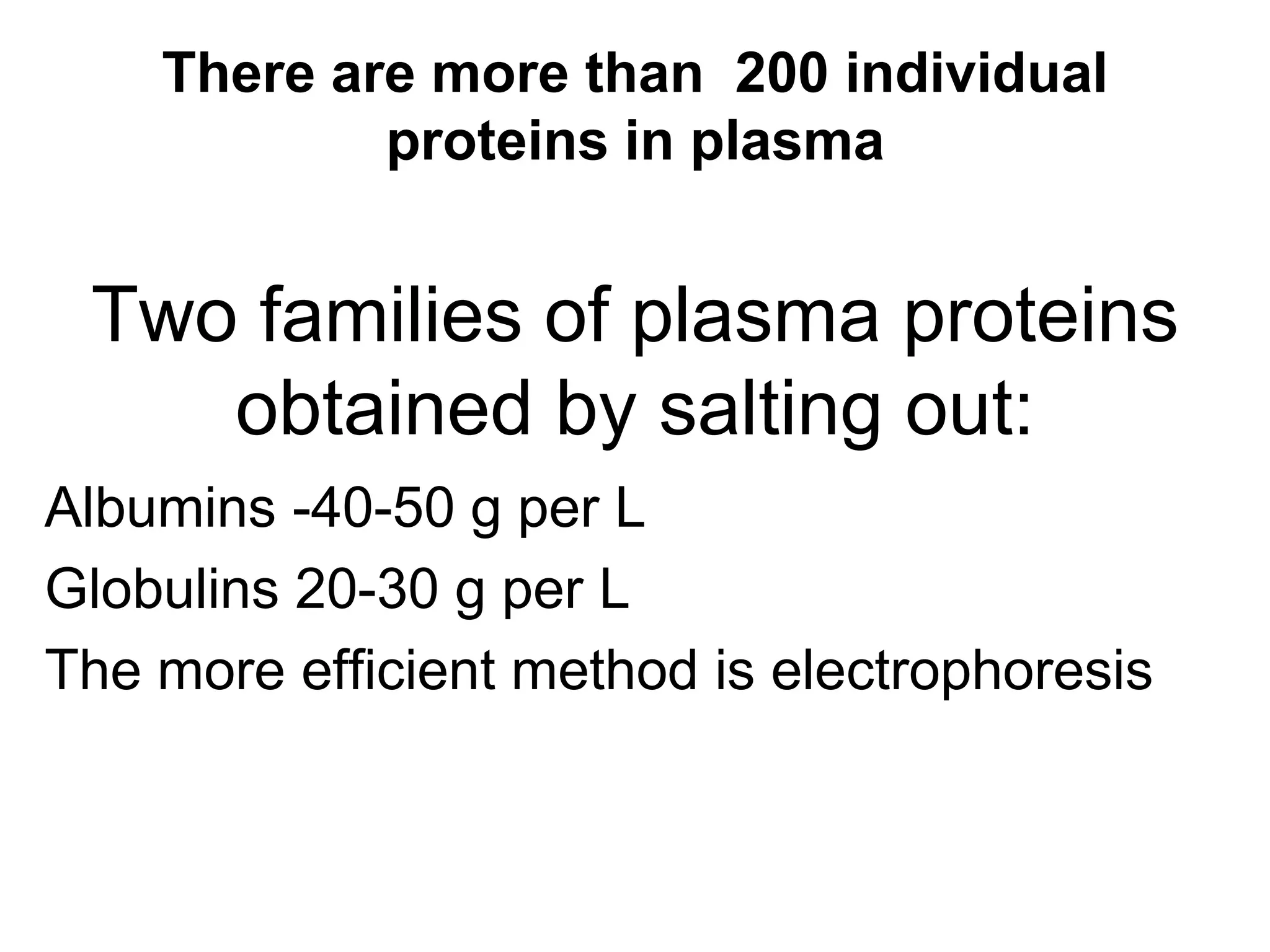
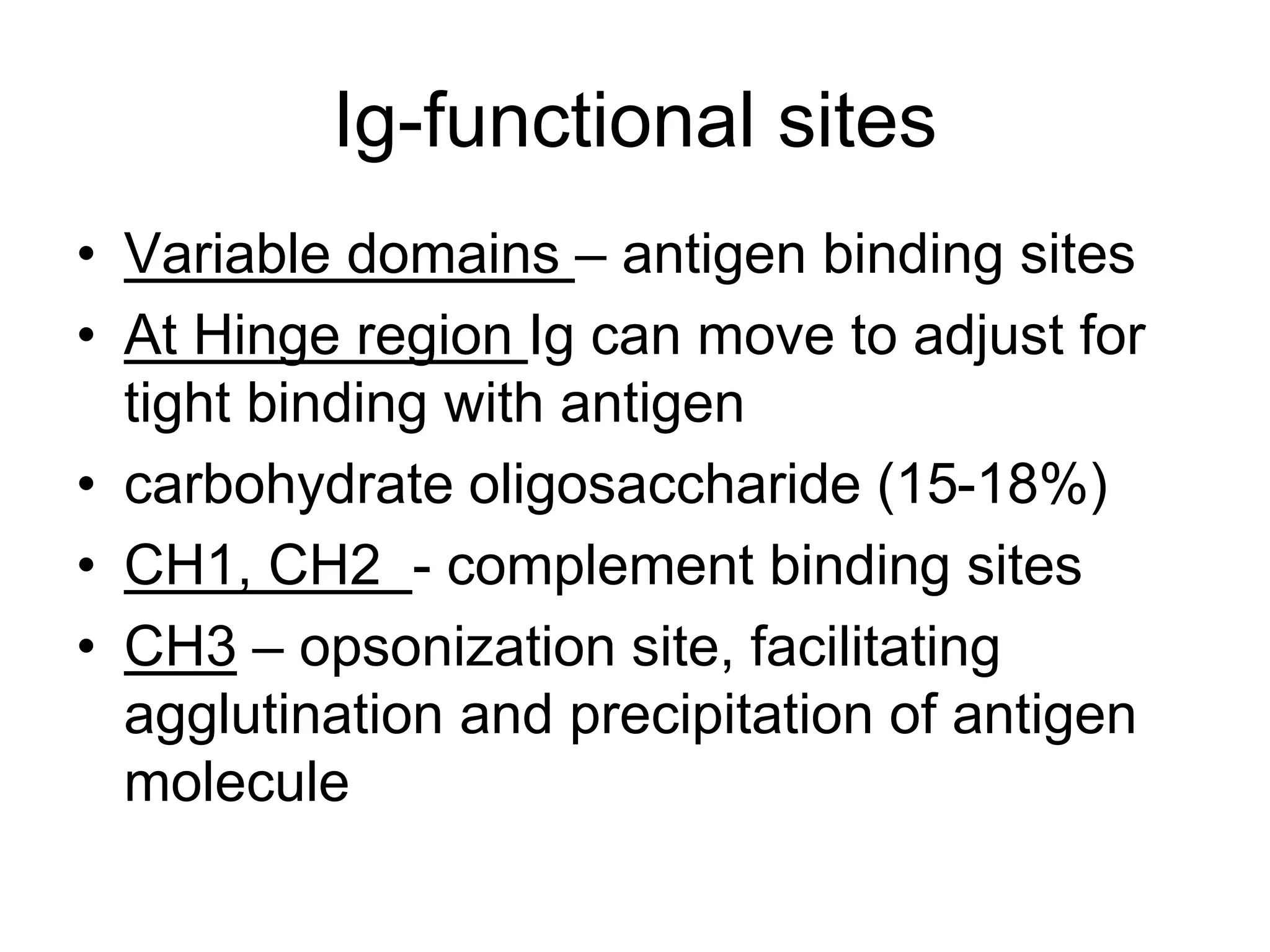
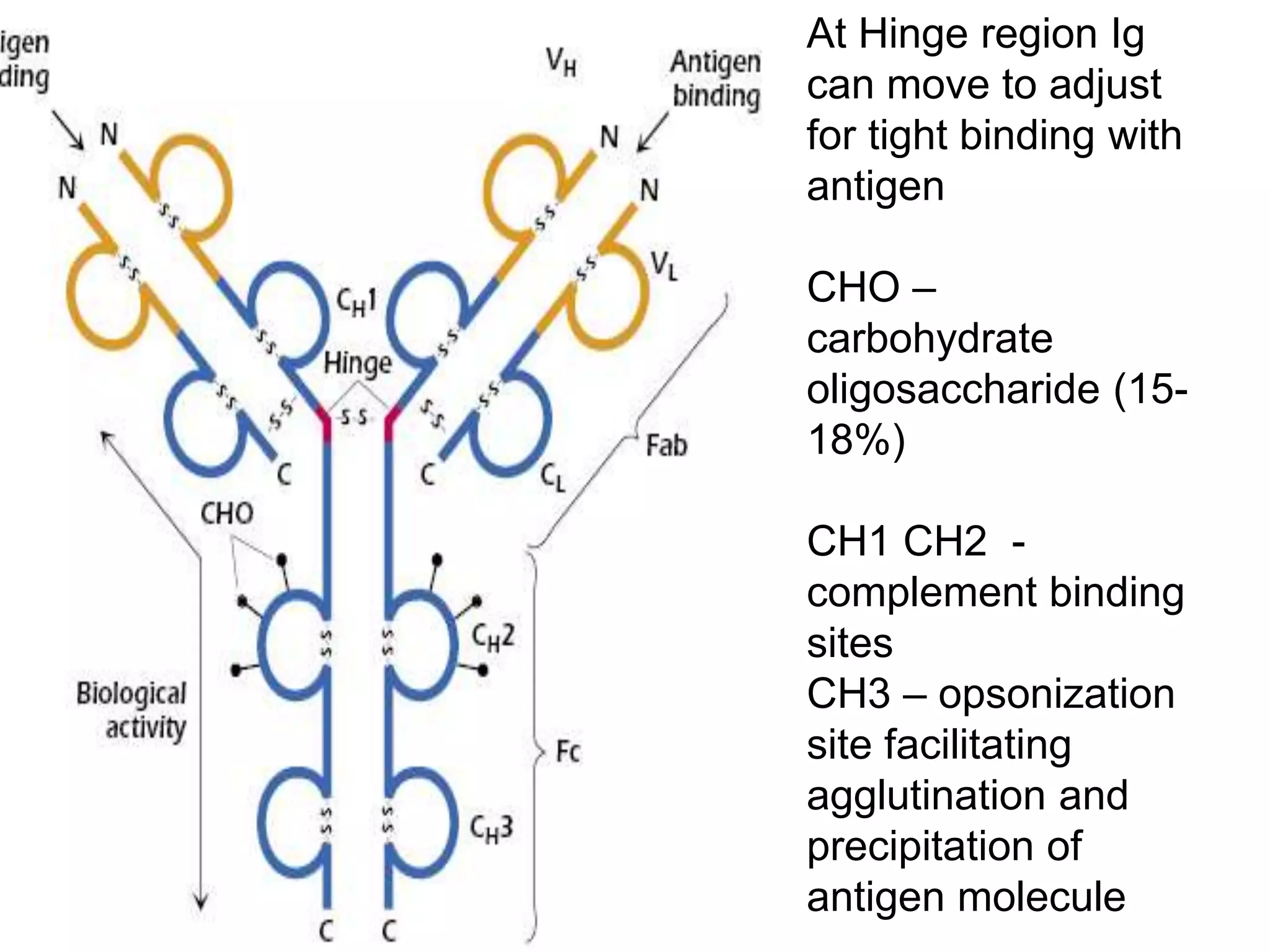
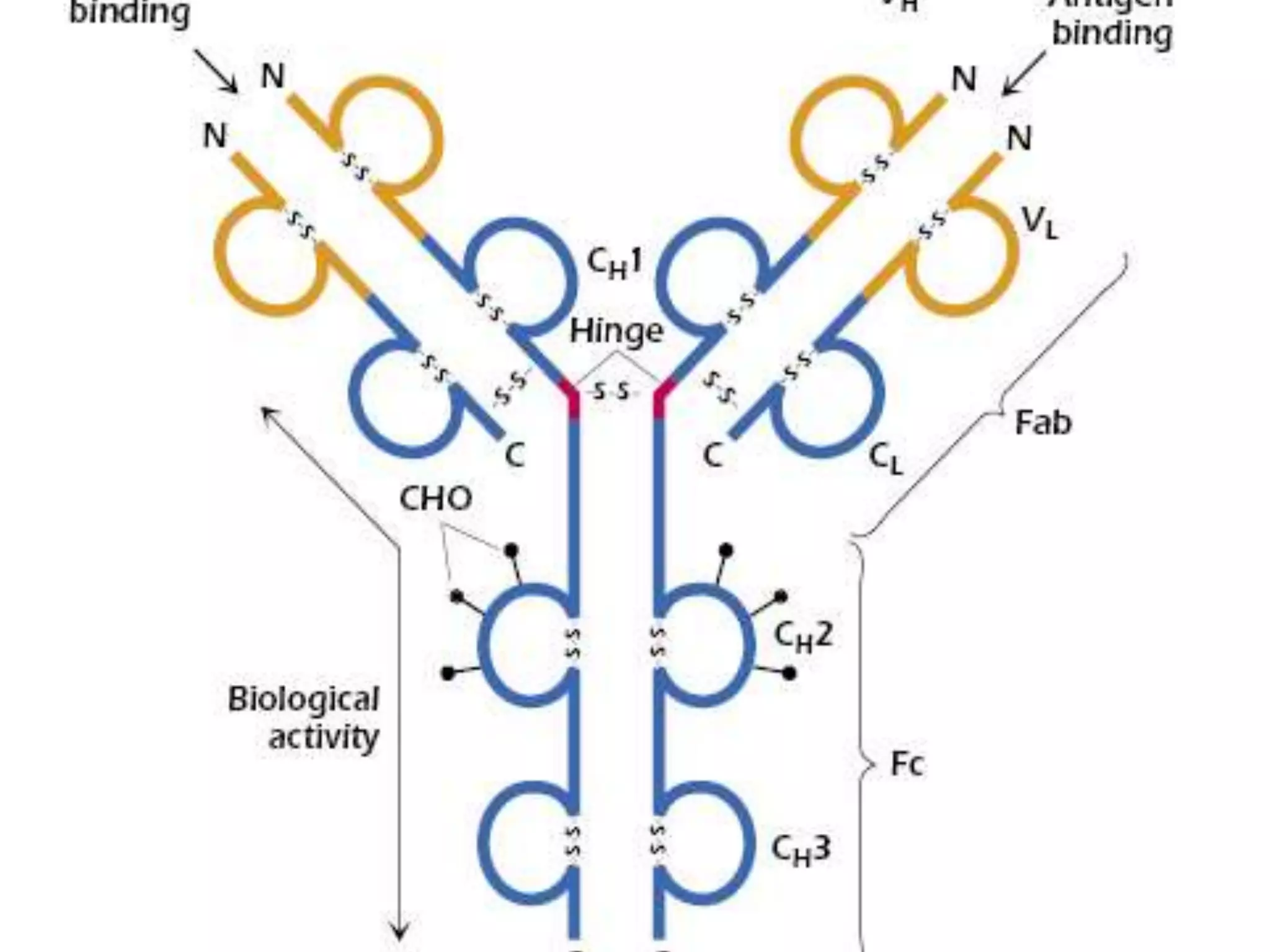
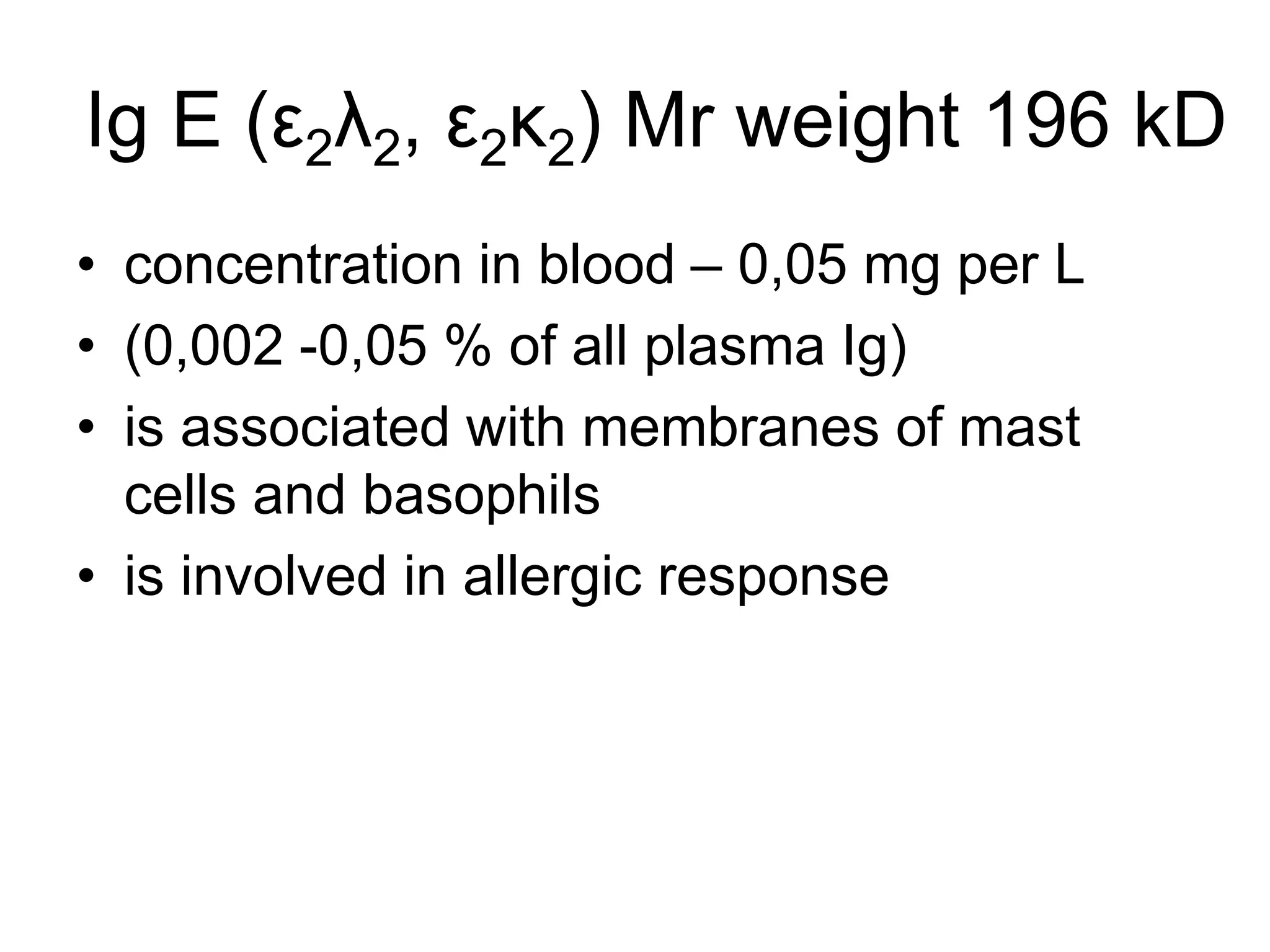
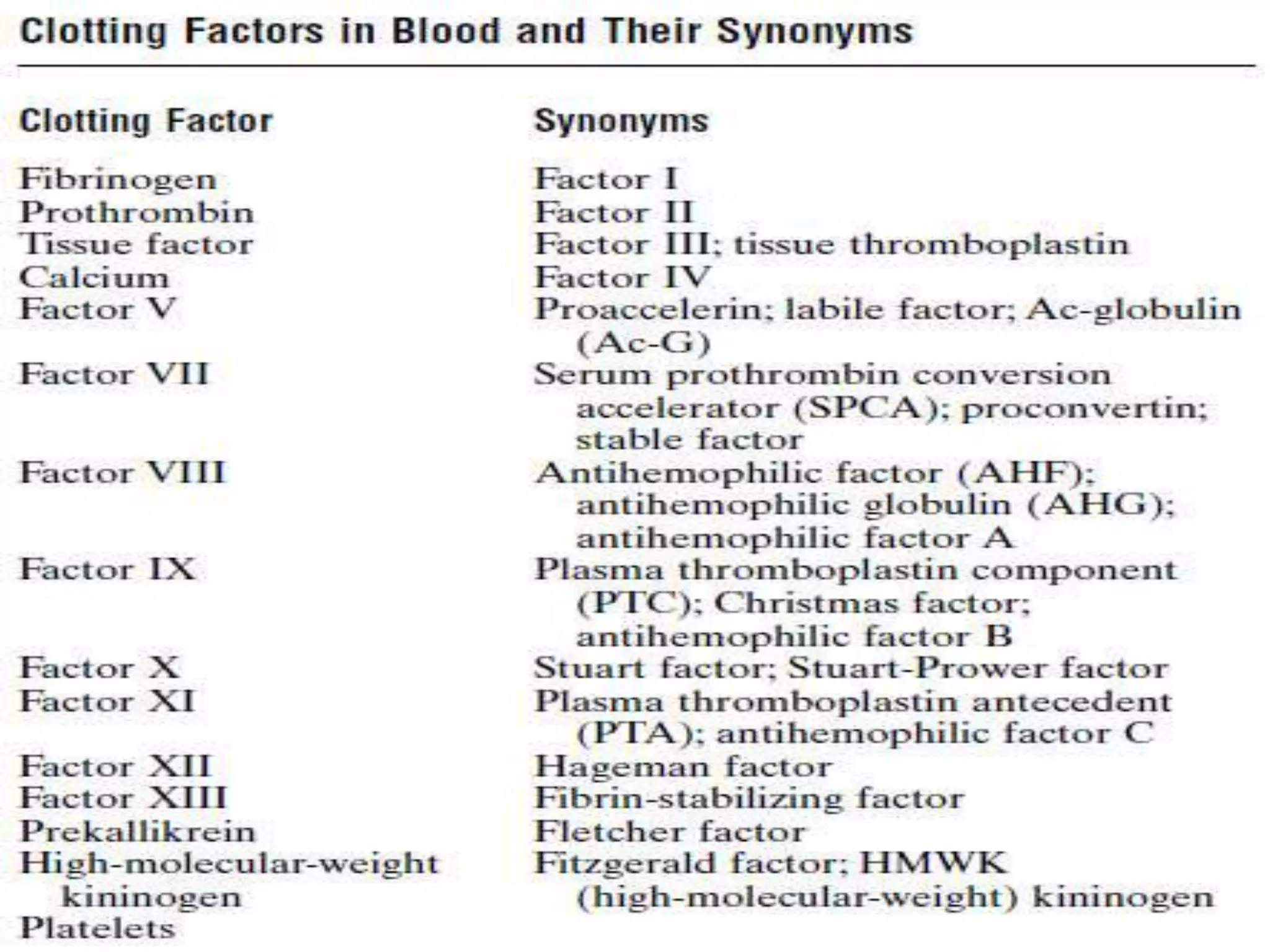
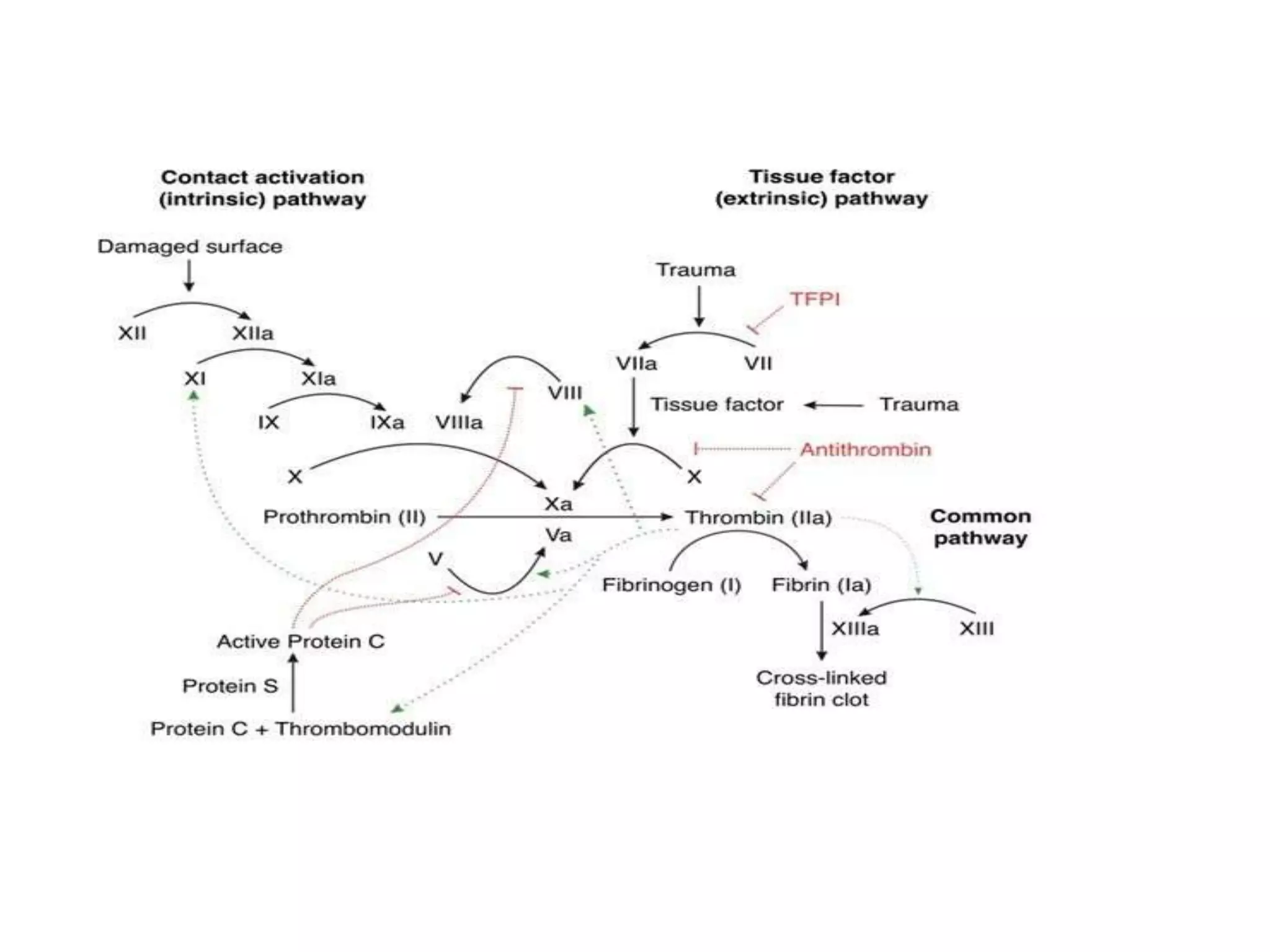
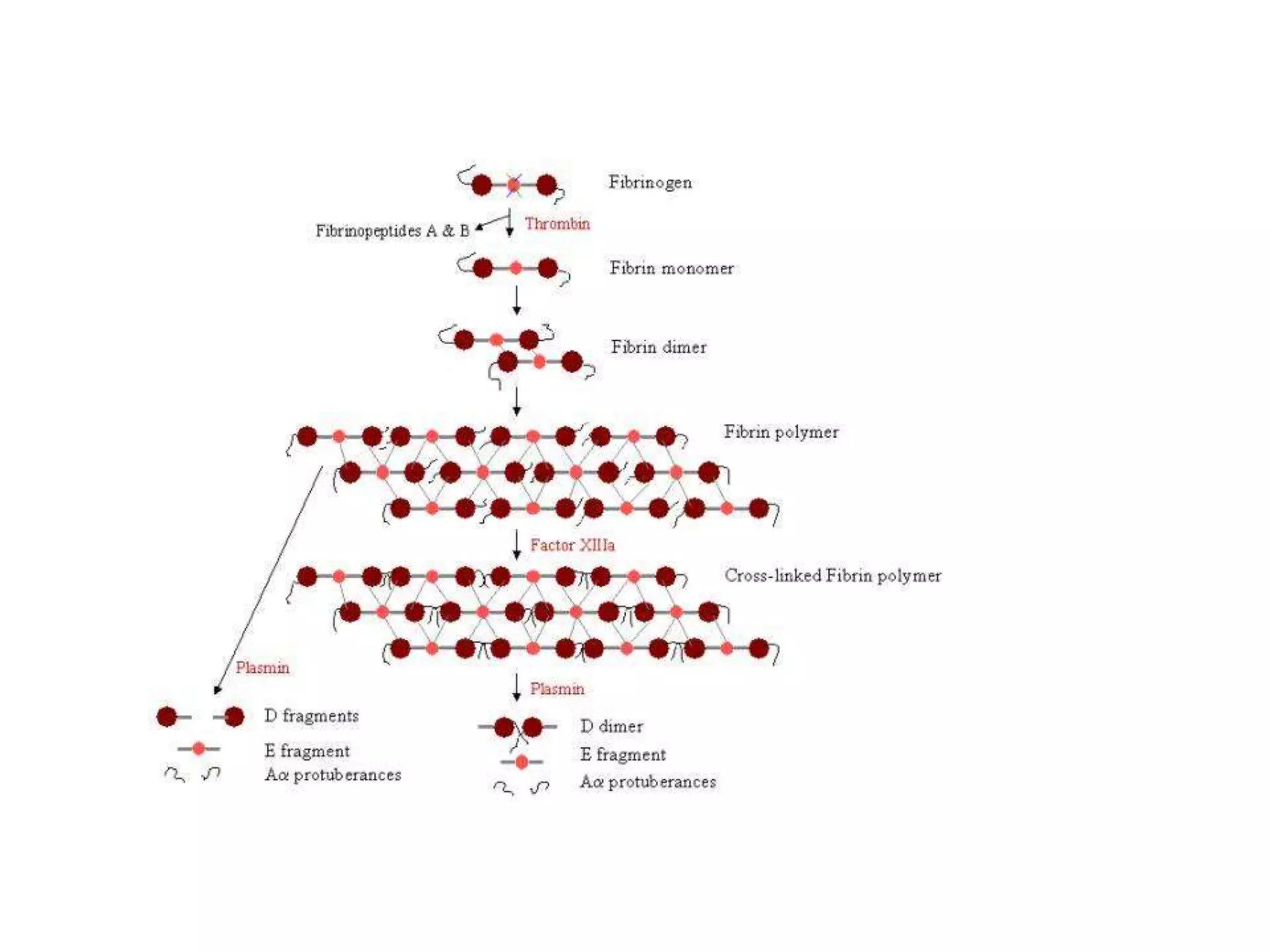
This document discusses the composition and functions of blood plasma proteins. It notes that plasma contains over 200 individual proteins that can be categorized into albumins and globulins. The major globulin categories are alpha-1, alpha-2, beta, and gamma globulins. Gamma globulins contain immunoglobulins which are antibodies that bind to antigens. The document describes the five major immunoglobulin classes - IgA, IgG, IgM, IgD, and IgE - and their properties and functions in the immune system. It also discusses other important plasma proteins like fibrinogen, complement proteins, acute phase proteins, transport proteins, and enzymes.