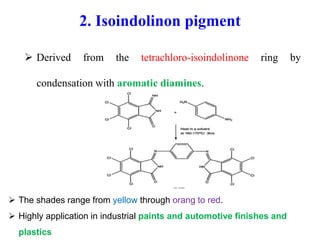
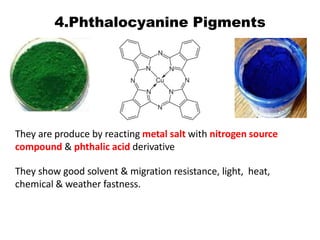
This document provides information about pigments, including:
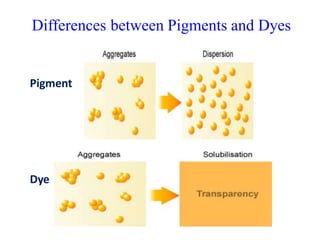
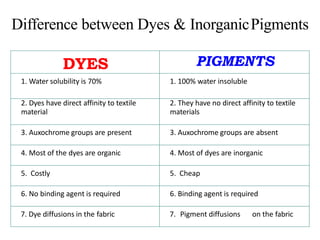
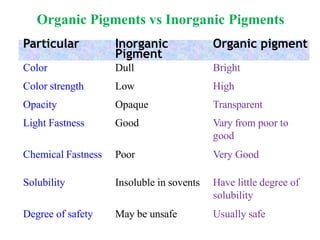
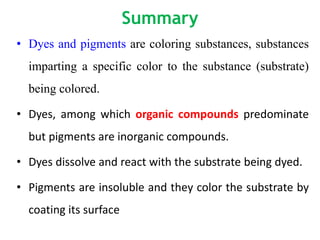
- Pigments are insoluble coloring substances that are used to color materials without chemically bonding to them.
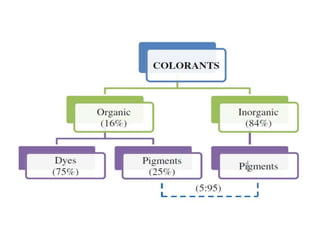
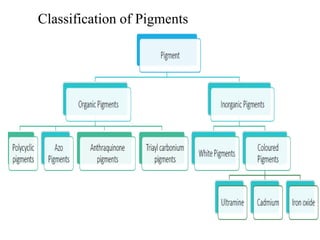
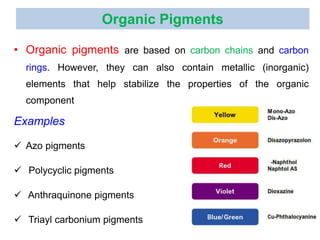
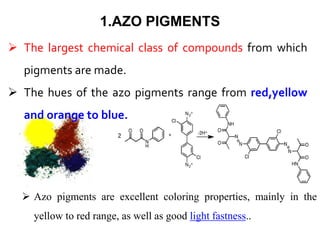
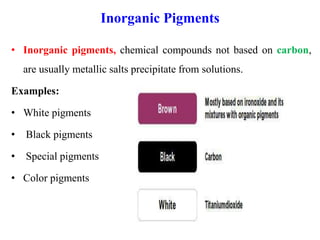
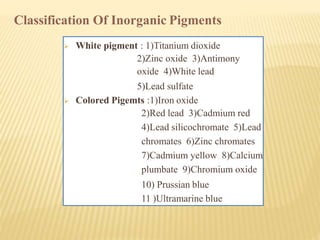
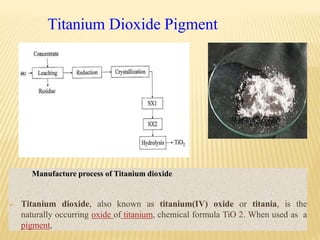
- Organic pigments include azo, polycyclic, and phthalocyanine pigments. Inorganic pigments include titanium dioxide, iron oxides, cadmium and chromium pigments.
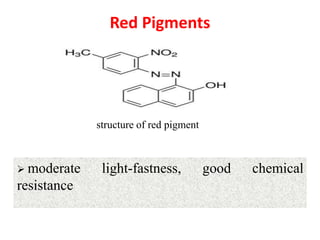
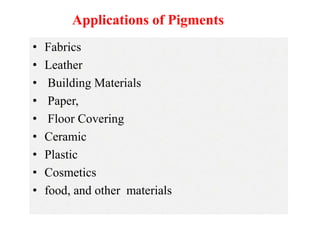
- Pigments are used to color fabrics, leather, paper, plastics, paints and other materials. They provide properties like color strength, light fastness and weather resistance.