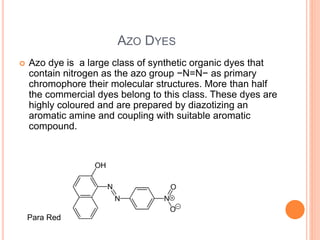
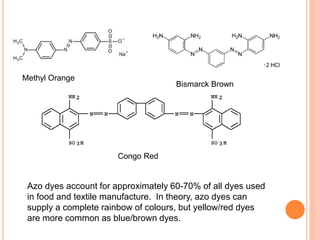
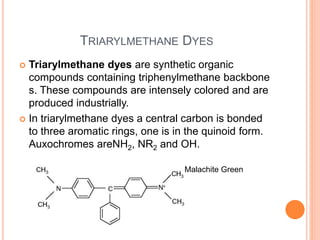
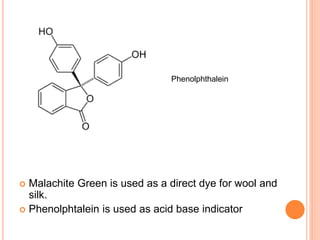
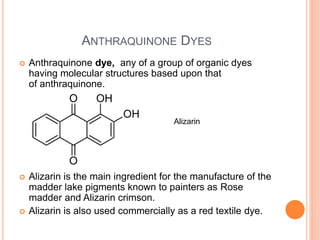
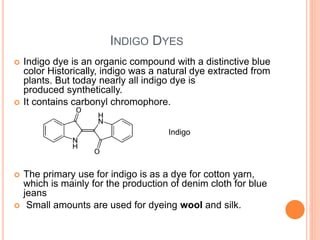
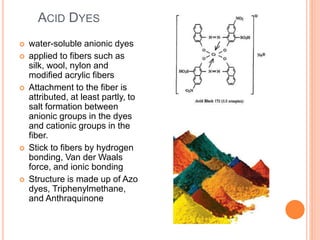
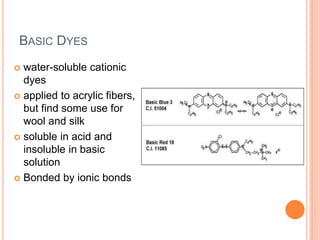
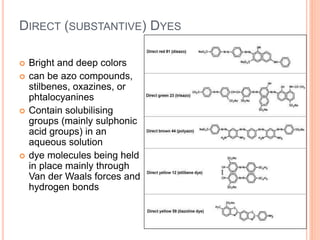
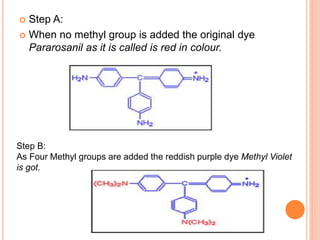
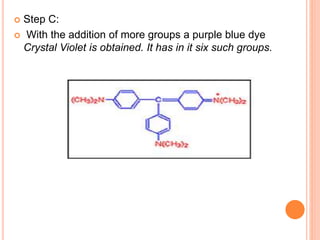
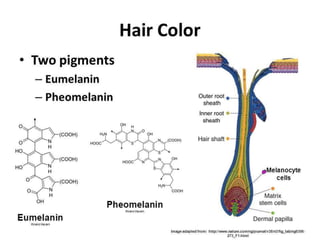
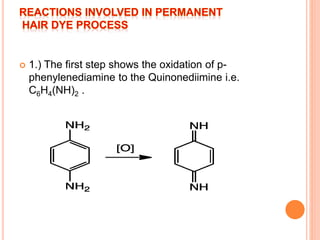
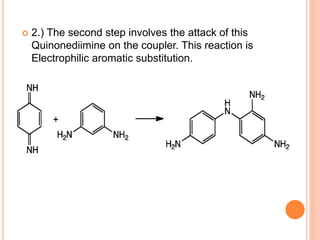
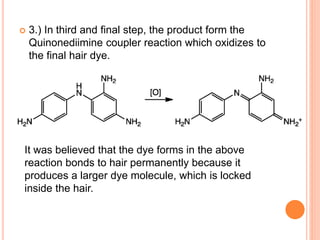
Muhammad Ahsan presented on synthetic dyes. He discussed that dyes can be classified based on their source as natural or synthetic dyes. Synthetic dyes, first created by William Henry Perkin in 1856, are now used more widely due to lower costs and greater color fastness. Dyes can also be classified by their chromophore, such as azo dyes containing the -N=N- group, or by application method like acid dyes which are water-soluble anionic dyes. Chromophores allow dyes to absorb visible light and appear colored. Modifiers to dye structures can alter their color by changing electron energies.