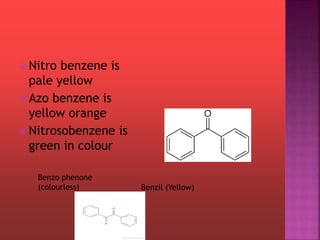
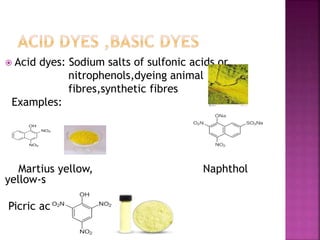
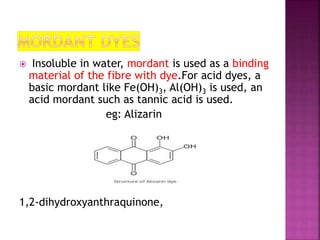
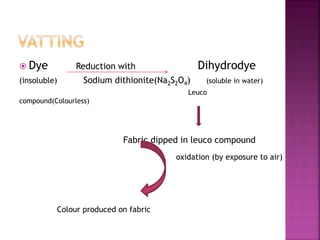
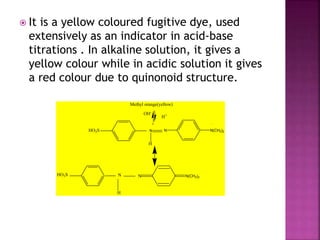
This document provides information about different types of dyes:
- Dyes can be classified based on their fastness properties as either "fast dyes" that are permanent or "fugitive dyes" that fade.
- Dyes are generally applied in an aqueous solution and sometimes require a mordant to improve their fastness on fibers.
- Major dye classifications include acid dyes, basic dyes, direct or substantive dyes, mordant dyes, vat dyes, azo dyes, and sulfur dyes. Azo dyes are one of the most important classes and are prepared through azo coupling reactions.
- Examples of natural dyes mentioned include henna, w